Abstract
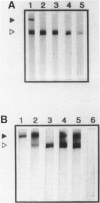
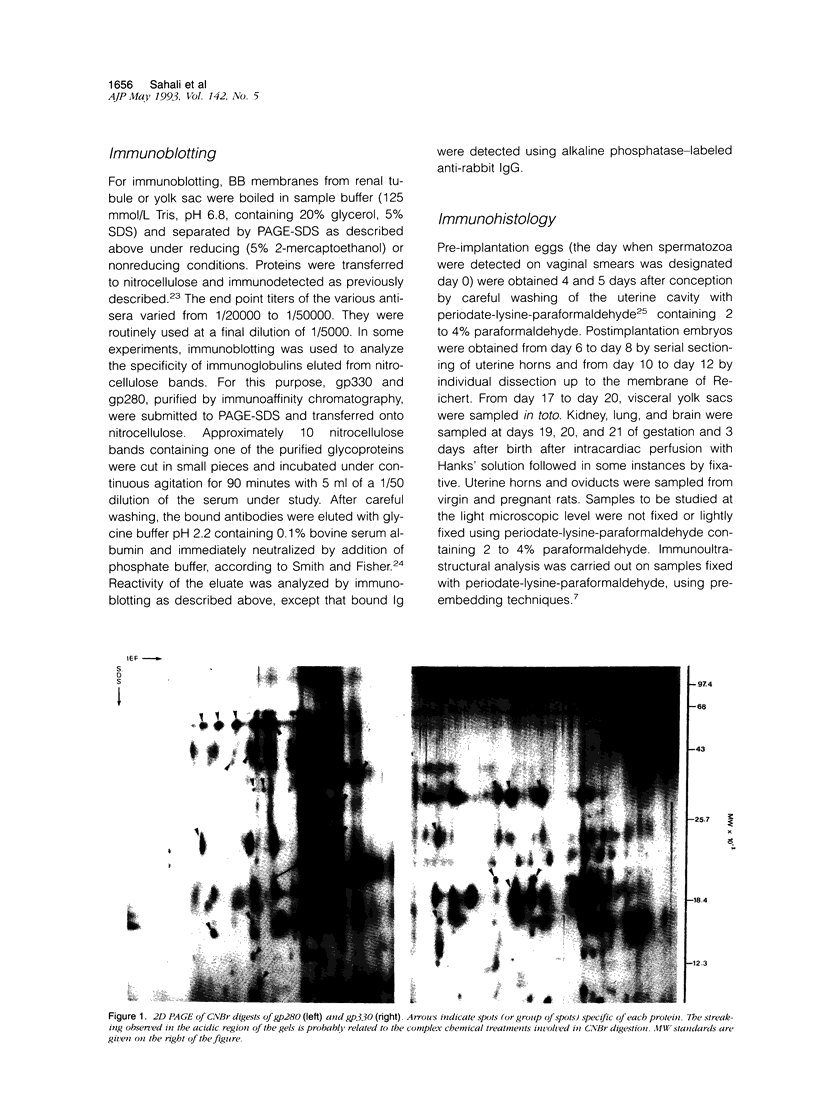
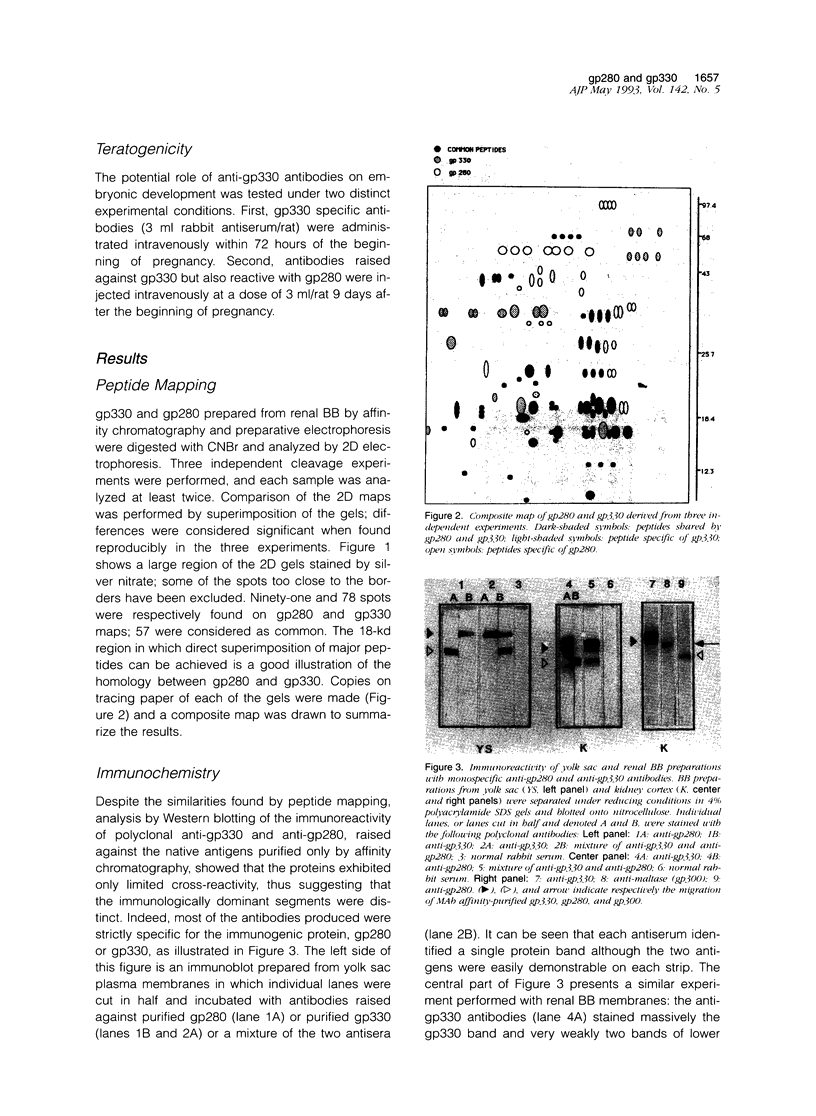
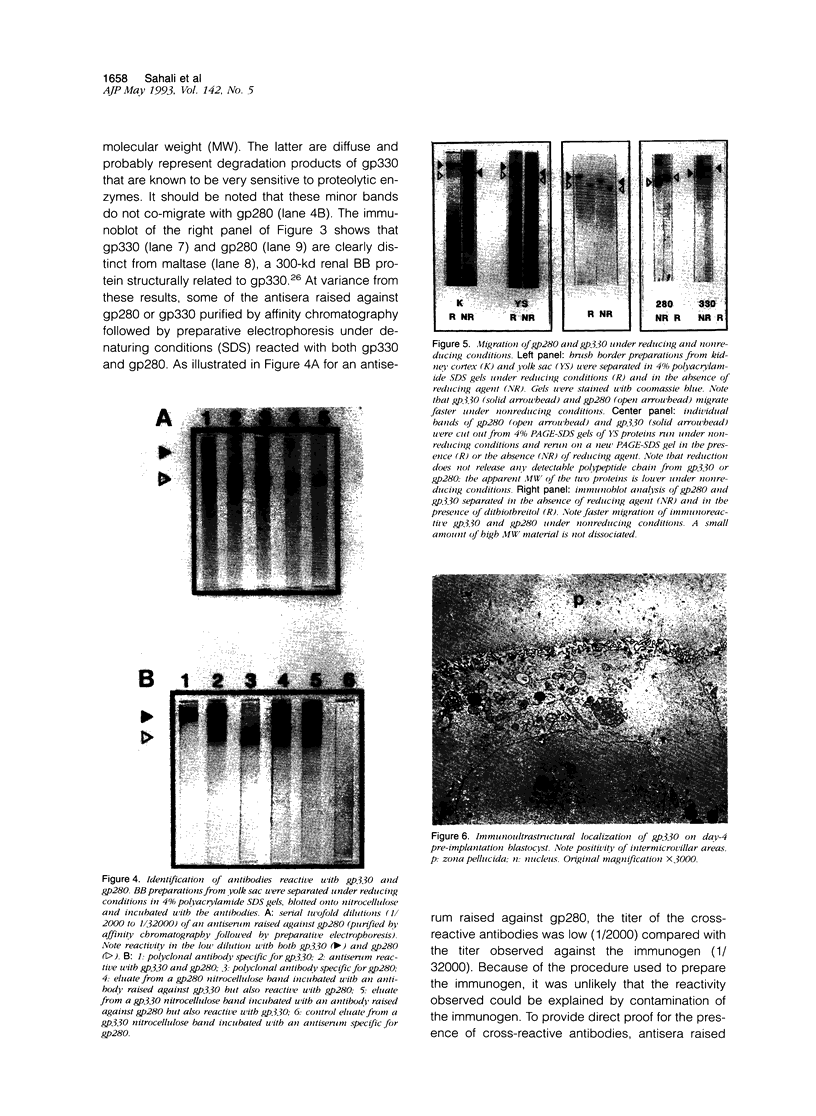
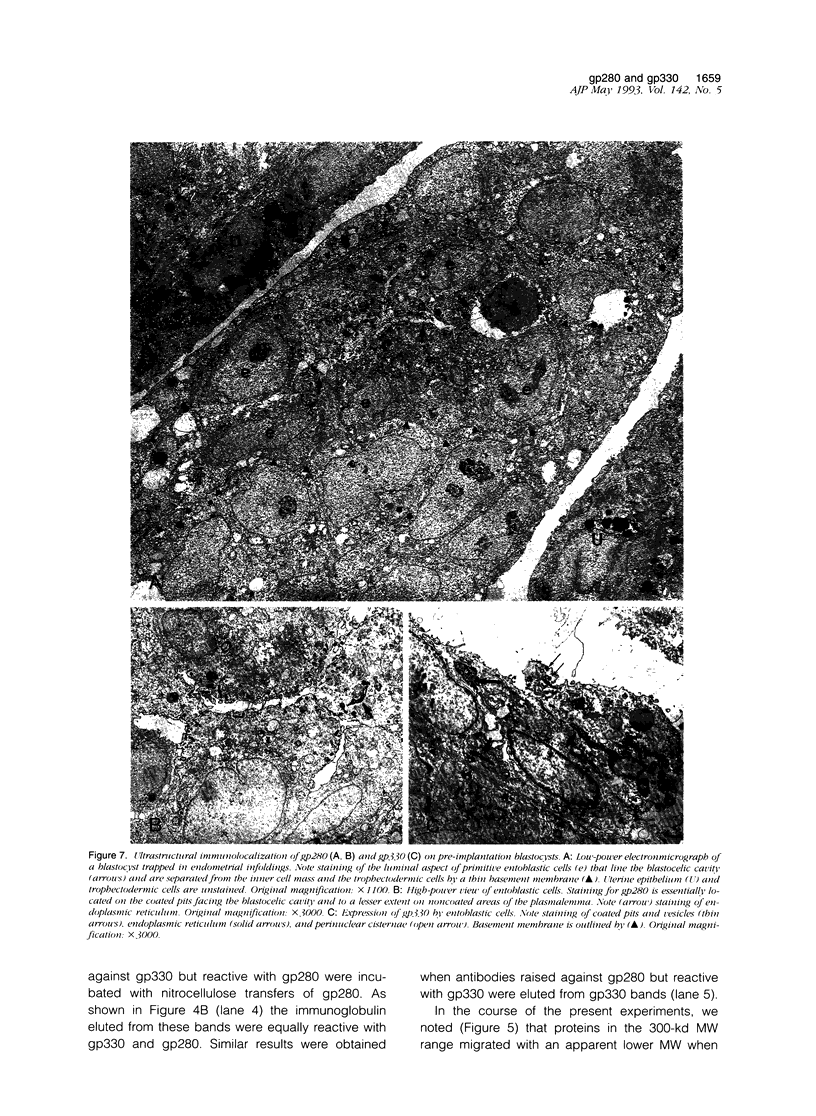
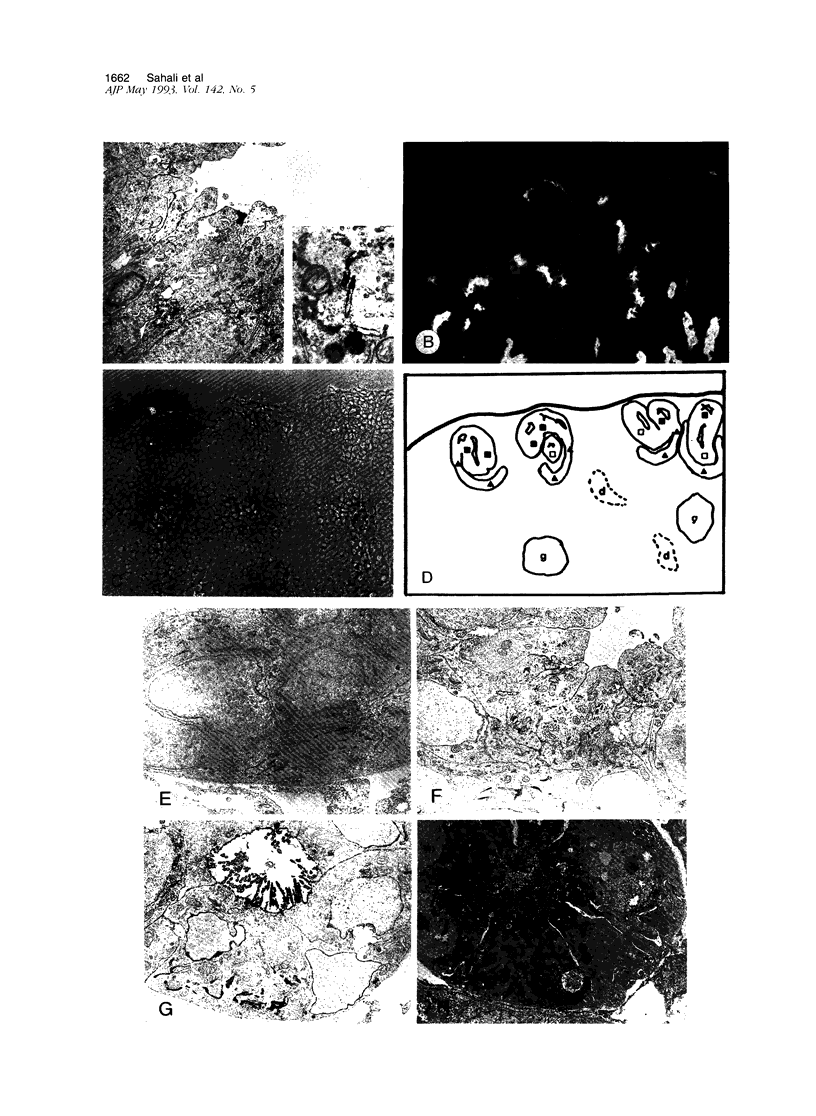
We have previously shown that monoclonal antibodies specific for a 280-kd protein (gp280) concentrated within the coated pits of renal and yolk sac brush border-induced fetal malformations, whereas antibodies specific for gp330, another coated pit protein with a similar distribution, had no deleterious effect on embryonic development. In this study, we show that gp280 and gp330 are closely related proteins, as indicated by: 1) similarities in peptide maps obtained after cyanogen bromide cleavage, 2) immunological cross-reactivity related to a minor contingent of antibodies that do not have teratogenic activity, and 3) asynchronous but related expressions during ontogenesis. During the early stages of development, the expression of the two glycoproteins was limited to (gp330) or predominant in (gp280) the clathrin-coated pits and intermicrovillar areas. In the pre-implantation embryo, gp330 was expressed by trophectodermal cells, which became negative in day-6 embryos trapped in endometrial infoldings. At this stage, gp280 and gp330 were both simultaneously detectable at the apical pole of the first entoblastic cells and remained expressed by the brush border of visceral yolk sac epithelial cells until the end of pregnancy. In addition, gp330 was expressed by amniotic cells and neurectodermal structures. During nephrogenesis, in contrast, the expression of gp280 and gp330 by the intermicrovillar areas of the proximal tubule cell was the result of a complex maturation process. gp280 and gp330 were diffusely distributed in S-shaped bodies in the presumptive areas of the glomerulus, proximal tubule, and distal tubule (gp330). During development of the nephron, the pattern of expression became progressively restricted to the proximal tubule and glomerulus (gp330), and selective localization in the intermicrovillar areas was only achieved in filtrating nephrons.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemesderfer D., Dekan G., Aronson P. S., Farquhar M. G. Assembly of distinctive coated pit and microvillar microdomains in the renal brush border. Am J Physiol. 1992 Jan;262(1 Pt 2):F55–F67. doi: 10.1152/ajprenal.1992.262.1.F55. [DOI] [PubMed] [Google Scholar]
- Brent R. L., Johnson A. J., Jensen M. The production of congenital malformations using tissue antisera. VII. Yolk-sac antiserum. Teratology. 1971 Aug;4(3):255–275. doi: 10.1002/tera.1420040302. [DOI] [PubMed] [Google Scholar]
- Brent R. L. Production of congenital malformations using tissue antisera. 3. Placental antiserum. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1024–1029. doi: 10.3181/00379727-125-32267. [DOI] [PubMed] [Google Scholar]
- Buc-Caron M. H., Condamine H., Kerjaschki D. Rat Heymann nephritis antigen is closely related to brushin, a glycoprotein present in early mouse embryo epithelia. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):707–722. doi: 10.1016/s0769-2625(87)80026-0. [DOI] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol. 1986 Mar;122(3):500–511. [PMC free article] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. II. Extrarenal distribution. Am J Pathol. 1986 Mar;122(3):512–519. [PMC free article] [PubMed] [Google Scholar]
- Clayman M. D., Sun M. J., Michaud L., Brill-Dashoff J., Riblet R., Neilson E. G. Clonotypic heterogeneity in experimental interstitial nephritis. Restricted specificity of the anti-tubular basement membrane B cell repertoire is associated with a disease-modifying crossreactive idiotype. J Exp Med. 1988 Apr 1;167(4):1296–1312. doi: 10.1084/jem.167.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID G., MERCIER-PAROT L., TUCHMANN-DUPLESSIS H. ACTION T'ERATOG'ENE D'H'ET'ERO-ANTICORPS TISSULAIRES. I. PRODUCTION DE MALFORMATIONS CHEZ LE RAT PAR ACTION D'UN S'ERUM ANTI-REIN) (FR) C R Seances Soc Biol Fil. 1963 Oct 5;157:939–942. [PubMed] [Google Scholar]
- Freeman S. J., Beck F., Lloyd J. B. The role of the visceral yolk sac in mediating protein utilization by rat embryos cultured in vitro. J Embryol Exp Morphol. 1981 Dec;66:223–234. [PubMed] [Google Scholar]
- Freeman S. J., Brent R. L., Lloyd J. B. The effect of teratogenic antiserum on yolk-sac function in rat embryos cultured in vitro. J Embryol Exp Morphol. 1982 Oct;71:63–74. [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J Cell Biol. 1991 May;113(4):731–741. doi: 10.1083/jcb.113.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Noronha-Blob L., Sacktor B., Farquhar M. G. Microdomains of distinctive glycoprotein composition in the kidney proximal tubule brush border. J Cell Biol. 1984 Apr;98(4):1505–1513. doi: 10.1083/jcb.98.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. The pathogenesis of membranous glomerulonephritis: from morphology to molecules. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58(4):253–271. doi: 10.1007/BF02890080. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung C. C., Cheewatrakoolpong B., Yan C. L. Teratogenic antibodies are directed against a coated-pit glycoprotein. Anat Rec. 1989 Apr;223(4):363–367. doi: 10.1002/ar.1092230403. [DOI] [PubMed] [Google Scholar]
- Leung C. C. Isolation, partial characterization, and localization of a rat renal tubular glycoprotein antigen. Antibody-induced birth defects. J Exp Med. 1982 Aug 1;156(2):372–384. doi: 10.1084/jem.156.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. C., Lee C., Cheewatrakoolpong B., Hilton D. Abnormal embryonic development induced by antibodies to rat visceral yolk-sac endoderm: isolation of the antigen and localization to microvillar membrane. Dev Biol. 1985 Feb;107(2):432–441. doi: 10.1016/0012-1606(85)90325-2. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Pietromonaco S., Kerjaschki D., Binder S., Ullrich R., Farquhar M. G. Molecular cloning of a cDNA encoding a major pathogenic domain of the Heymann nephritis antigen gp330. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1811–1815. doi: 10.1073/pnas.87.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey C. D., Dash A., Kershaw M. J., Morgan A., Reilly A., Rees A. J., Lockwood C. M. A single autoantigen in Goodpasture's syndrome identified by a monoclonal antibody to human glomerular basement membrane. Lab Invest. 1987 Jan;56(1):23–31. [PubMed] [Google Scholar]
- Rodman J. S., Kerjaschki D., Merisko E., Farquhar M. G. Presence of an extensive clathrin coat on the apical plasmalemma of the rat kidney proximal tubule cell. J Cell Biol. 1984 May;98(5):1630–1636. doi: 10.1083/jcb.98.5.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Melcion C., Geniteau M., Ronco E., Reininger L., Galceran M., Verroust P. Production and characterization of monoclonal antibodies against rat brush border antigens of the proximal convoluted tubule. Immunology. 1984 Sep;53(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Neale T. J., Wilson C. B., Galceran M., Verroust P. An immunopathologic study of a 330-kD protein defined by monoclonal antibodies and reactive with anti-RTE alpha 5 antibodies and kidney eluates from active Heymann nephritis. J Immunol. 1986 Jan;136(1):125–130. [PubMed] [Google Scholar]
- Sahali D., Mulliez N., Chatelet F., Dupuis R., Ronco P., Verroust P. Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med. 1988 Jan 1;167(1):213–218. doi: 10.1084/jem.167.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahali D., Mulliez N., Chatelet F., Laurent-Winter C., Citadelle D., Roux C., Ronco P., Verroust P. Coexpression in humans by kidney and fetal envelopes of a 280 kDa-coated pit-restricted protein. Similarity with the murine target of teratogenic antibodies. Am J Pathol. 1992 Jan;140(1):33–44. [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. I. Kinetics of uptake of (125I)polyvinylpyrrolidone by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):113–122. doi: 10.1083/jcb.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]