Abstract
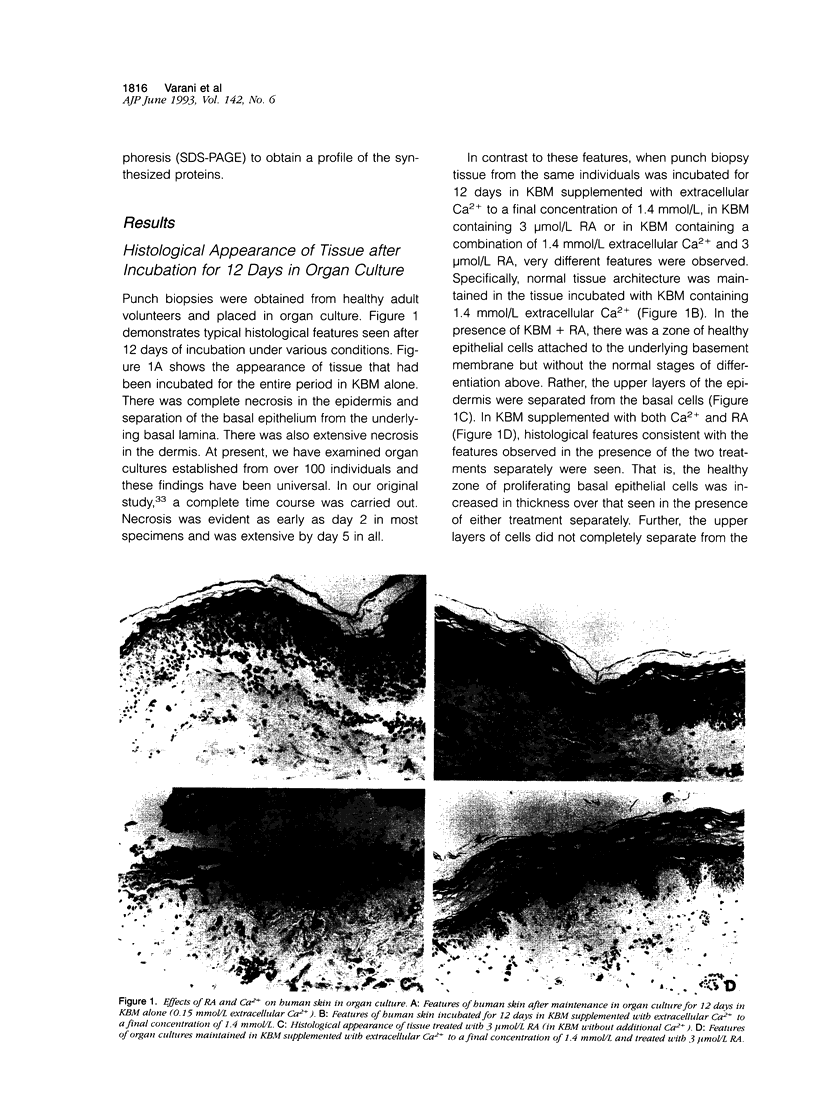
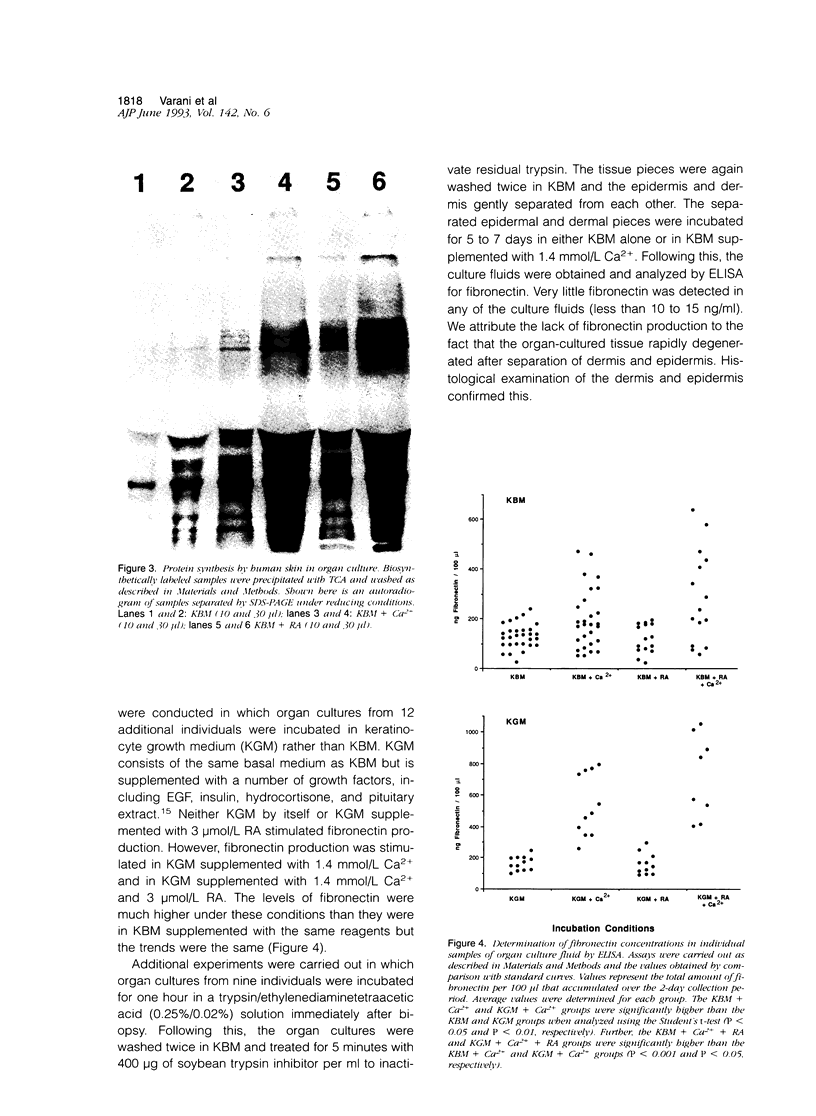
Two-mm full-thickness punch biopsies of human skin were placed in organ culture in a serum-free, growth factor-free basal medium. Under conditions of low extracellular Ca2+ (0.15 mmol/L), the tissue quickly degenerated. However, degeneration was prevented when the extracellular Ca2+ concentration was increased to 1.4 mmol/L. The tissue remained histologically normal in appearance and biochemically active for up to 12 days. The addition of 3 mumol/L all-trans retinoic acid (RA) to the low-Ca2+ culture medium also prevented tissue degeneration. However, in contrast to what was seen in the presence of 1.4 mmol/L Ca2+, epidermal differentiation did not occur normally in the presence of RA. Rather, the upper layers of the epidermis routinely separated from the underlying basal cells. Fibronectin production by the organ cultured skin was examined. Biosynthetic labeling/immunoprecipitation studies demonstrated that incubation of the tissue in basal medium containing 1.4 mmol/L Ca2+ resulted in a high level of fibronectin production relative to the amount produced in basal medium containing 0.15 mmol/L Ca2+. In contrast, the addition of 3 mumol/L RA to the low Ca2+ basal medium did not stimulate fibronectin production. Similar results were observed in enzyme-linked immunosorbent assays where the addition of Ca2+ to a final concentration of 1.4 mmol/L stimulated fibronectin and thrombospondin production whereas RA (3 mumol/L) did not. Although RA by itself failed to stimulate extracellular matrix production, the addition of 3 mumol/L RA to basal medium containing 1.4 mmol/L Ca2+ led to a further increase in fibronectin production over that seen in the presence of 1.4 mmol/L Ca2+ alone. Taken together, these data indicate that although either 1.4 mmol/L Ca2+ or 3 mumol/L RA facilitates survival of organ-cultured skin in basal medium, they have very different effects on extracellular matrix production. This supports the view, based on histological appearance, that the two treatments work through different mechanisms. The data further support the suggestion that the two treatments may have additive or even synergistic effects.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barreca A., De Luca M., Del Monte P., Bondanza S., Damonte G., Cariola G., Di Marco E., Giordano G., Cancedda R., Minuto F. In vitro paracrine regulation of human keratinocyte growth by fibroblast-derived insulin-like growth factors. J Cell Physiol. 1992 May;151(2):262–268. doi: 10.1002/jcp.1041510207. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S., Brattain M. G., Ochs R. L., Varani J. Modulation of fibronectin, laminin, and cellular adhesion in the transformation and differentiation of murine AKR fibroblasts. J Cell Physiol. 1987 Dec;133(3):415–425. doi: 10.1002/jcp.1041330302. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S., Jan Y., Levine A., McClenic B., Varani J. Fibronectin/laminin and their receptors in aberrant growth control in FR3T3 cells transformed by Ha-ras oncogene and epidermal growth factor gene. Int J Cancer. 1989 Aug 15;44(2):325–331. doi: 10.1002/ijc.2910440223. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Nielsen L. D., Howell S. E., Folkvord J. M. Human keratinocytes that have not terminally differentiated synthesize laminin and fibronectin but deposit only fibronectin in the pericellular matrix. J Cell Biochem. 1985;28(2):127–141. doi: 10.1002/jcb.240280206. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Winn H. J., Dvorak H. F., Colvin R. B. Fibronectin beneath reepithelializing epidermis in vivo: sources and significance. J Invest Dermatol. 1983 Jun;80 (Suppl):26s–30s. [PubMed] [Google Scholar]
- Colige A., Nusgens B., Lapiere C. M. Response to epidermal growth factor of skin fibroblasts from donors of varying age is modulated by the extracellular matrix. J Cell Physiol. 1990 Dec;145(3):450–457. doi: 10.1002/jcp.1041450309. [DOI] [PubMed] [Google Scholar]
- Cook P. W., Mattox P. A., Keeble W. W., Pittelkow M. R., Plowman G. D., Shoyab M., Adelman J. P., Shipley G. D. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991 May;11(5):2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. W., Pittelkow M. R., Shipley G. D. Growth factor-independent proliferation of normal human neonatal keratinocytes: production of autocrine- and paracrine-acting mitogenic factors. J Cell Physiol. 1991 Feb;146(2):277–289. doi: 10.1002/jcp.1041460213. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989 Mar;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Ferriola P. C., Earp H. S., Di Augustine R., Nettesheim P. Role of TGF alpha and its receptor in proliferation of immortalized rat tracheal epithelial cells: studies with tyrphostin and TGF alpha antisera. J Cell Physiol. 1991 Apr;147(1):166–175. doi: 10.1002/jcp.1041470121. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette G. P., Carey T. E., Varani J., Schwartz D. R., Fligiel S. E., Ruddon R. W., Peters B. P. Biosynthesis and secretion of laminin and laminin-associated glycoproteins by nonmalignant and malignant human keratinocytes: comparison of cell lines from primary and secondary tumors in the same patient. Cancer Res. 1988 Sep 15;48(18):5193–5202. [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Harper R. A. Specificity in the synergism between retinoic acid and EGF on the growth of adult human skin fibroblasts. Exp Cell Res. 1988 Oct;178(2):254–263. doi: 10.1016/0014-4827(88)90396-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Kligman A. M., Grove G. L., Hirose R., Leyden J. J. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Duo C. H., Kligman A. M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):779-85, 884-7. doi: 10.1016/s0190-9622(86)70234-x. [DOI] [PubMed] [Google Scholar]
- Krane J. F., Murphy D. P., Carter D. M., Krueger J. G. Synergistic effects of epidermal growth factor (EGF) and insulin-like growth factor I/somatomedin C (IGF-I) on keratinocyte proliferation may be mediated by IGF-I transmodulation of the EGF receptor. J Invest Dermatol. 1991 Apr;96(4):419–424. doi: 10.1111/1523-1747.ep12469799. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Karasek M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J Invest Dermatol. 1978 Aug;71(2):157–162. doi: 10.1111/1523-1747.ep12546943. [DOI] [PubMed] [Google Scholar]
- Maciag T., Nemore R. E., Weinstein R., Gilchrest B. A. An endocrine approach to the control of epidermal growth: serum-free cultivation of human keratinocytes. Science. 1981 Mar 27;211(4489):1452–1454. doi: 10.1126/science.6970413. [DOI] [PubMed] [Google Scholar]
- Neely E. K., Morhenn V. B., Hintz R. L., Wilson D. M., Rosenfeld R. G. Insulin-like growth factors are mitogenic for human keratinocytes and a squamous cell carcinoma. J Invest Dermatol. 1991 Jan;96(1):104–110. doi: 10.1111/1523-1747.ep12515914. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Riser B. L., Mitra R. S., Dixit V. M., Varani J. Inhibitory effect of gamma interferon on cultured human keratinocyte thrombospondin production, distribution, and biologic activities. J Invest Dermatol. 1988 Sep;91(3):213–218. doi: 10.1111/1523-1747.ep12465005. [DOI] [PubMed] [Google Scholar]
- O'Keefe E. J., Chiu M. L. Stimulation of thymidine incorporation in keratinocytes by insulin, epidermal growth factor, and placental extract: comparison with cell number to assess growth. J Invest Dermatol. 1988 Jan;90(1):2–7. doi: 10.1111/1523-1747.ep12462409. [DOI] [PubMed] [Google Scholar]
- Pentland A. P., Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986 Jan;77(1):246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991 Jan;146(1):94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- Praeger F. C., Cristofalo V. J. Modulation of WI-38 cell proliferation by elevated levels of CaCl2. J Cell Physiol. 1986 Oct;129(1):27–35. doi: 10.1002/jcp.1041290105. [DOI] [PubMed] [Google Scholar]
- Ristow H. J. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be interleukin 1 or a related protein. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1940–1944. doi: 10.1073/pnas.84.7.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow H. J., Messmer T. O. Basic fibroblast growth factor and insulin-like growth factor I are strong mitogens for cultured mouse keratinocytes. J Cell Physiol. 1988 Nov;137(2):277–284. doi: 10.1002/jcp.1041370210. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Ziboh V. A., Isseroff R. R., Martinez D. Novel regulatory actions of 1 alpha,25-dihydroxyvitamin D3 on the metabolism of polyphosphoinositides in murine epidermal keratinocytes. J Cell Physiol. 1987 Jul;132(1):131–136. doi: 10.1002/jcp.1041320118. [DOI] [PubMed] [Google Scholar]
- Van Wyk J. J., Graves D. C., Casella S. J., Jacobs S. Evidence from monoclonal antibody studies that insulin stimulates deoxyribonucleic acid synthesis through the type I somatomedin receptor. J Clin Endocrinol Metab. 1985 Oct;61(4):639–643. doi: 10.1210/jcem-61-4-639. [DOI] [PubMed] [Google Scholar]
- Varani J., Astrom A., Griffiths C. E., Voorhees J. J. Induction of proliferation of growth-inhibited keratinocytes and fibroblasts in monolayer culture by sodium lauryl sulfate: comparison with all-trans retinoic acid. J Invest Dermatol. 1991 Nov;97(5):917–921. doi: 10.1111/1523-1747.ep12491682. [DOI] [PubMed] [Google Scholar]
- Varani J., Chakrabarty S. Modulation of fibronectin synthesis and fibronectin binding during transformation and differentiation of mouse AKR fibroblasts. J Cell Physiol. 1990 Jun;143(3):445–454. doi: 10.1002/jcp.1041430307. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Schuger L., Perone P., Inman D., Griffiths C. E., Voorhees J. J. Effects of all-trans retinoic acid and Ca++ on human skin in organ culture. Am J Pathol. 1993 Jan;142(1):189–198. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Gibbs D. F., Inman D. R., Shah B., Fligiel S. E., Voorhees J. J. Inhibition of epithelial cell adhesion by retinoic acid. Relationship to reduced extracellular matrix production and alterations in Ca2+ levels. Am J Pathol. 1991 Apr;138(4):887–895. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Mitra R. S., Gibbs D., Phan S. H., Dixit V. M., Mitra R., Jr, Wang T., Siebert K. J., Nickoloff B. J., Voorhees J. J. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990 May;94(5):717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Shayevitz J., Perry D., Mitra R. S., Nickoloff B. J., Voorhees J. J. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990 Jun;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]