Abstract
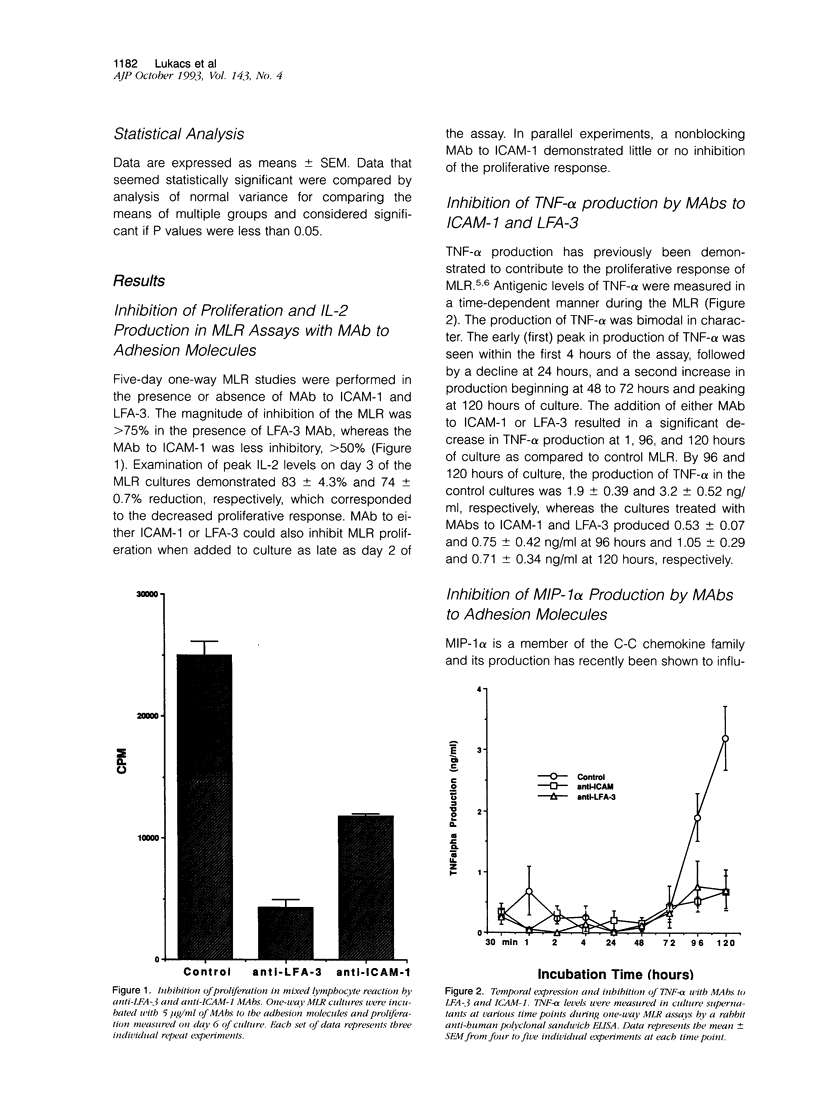
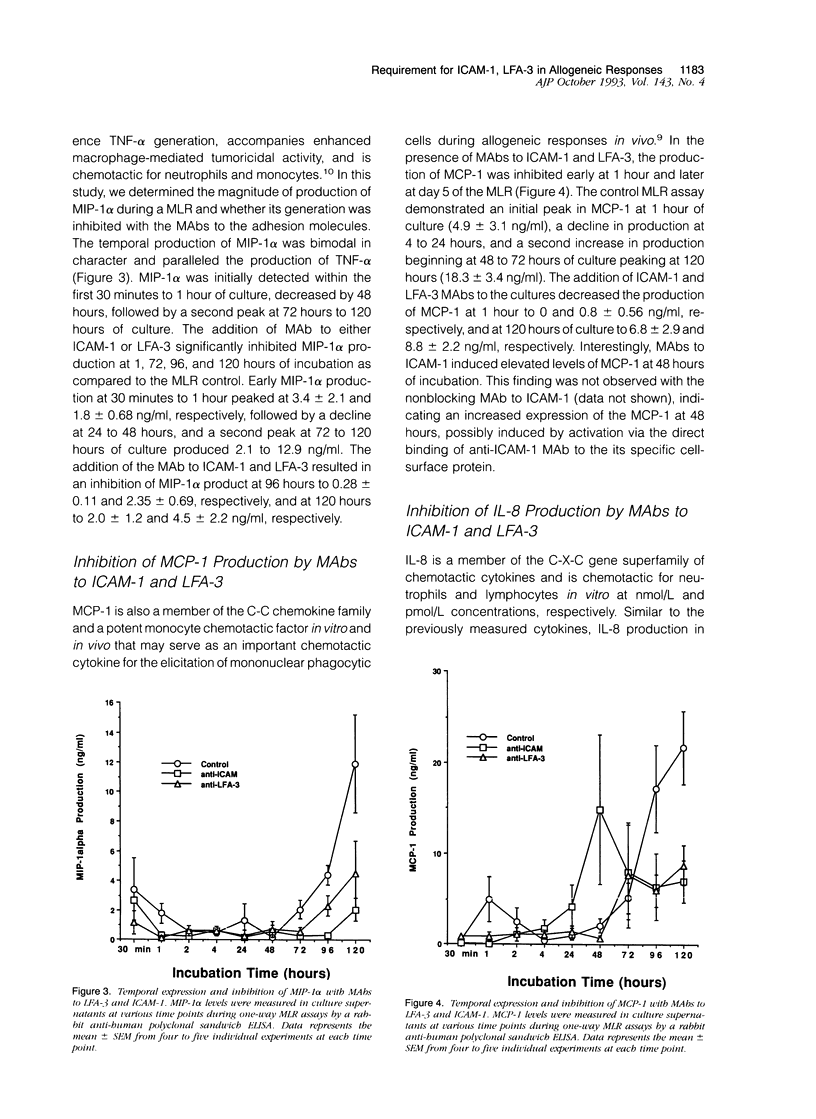
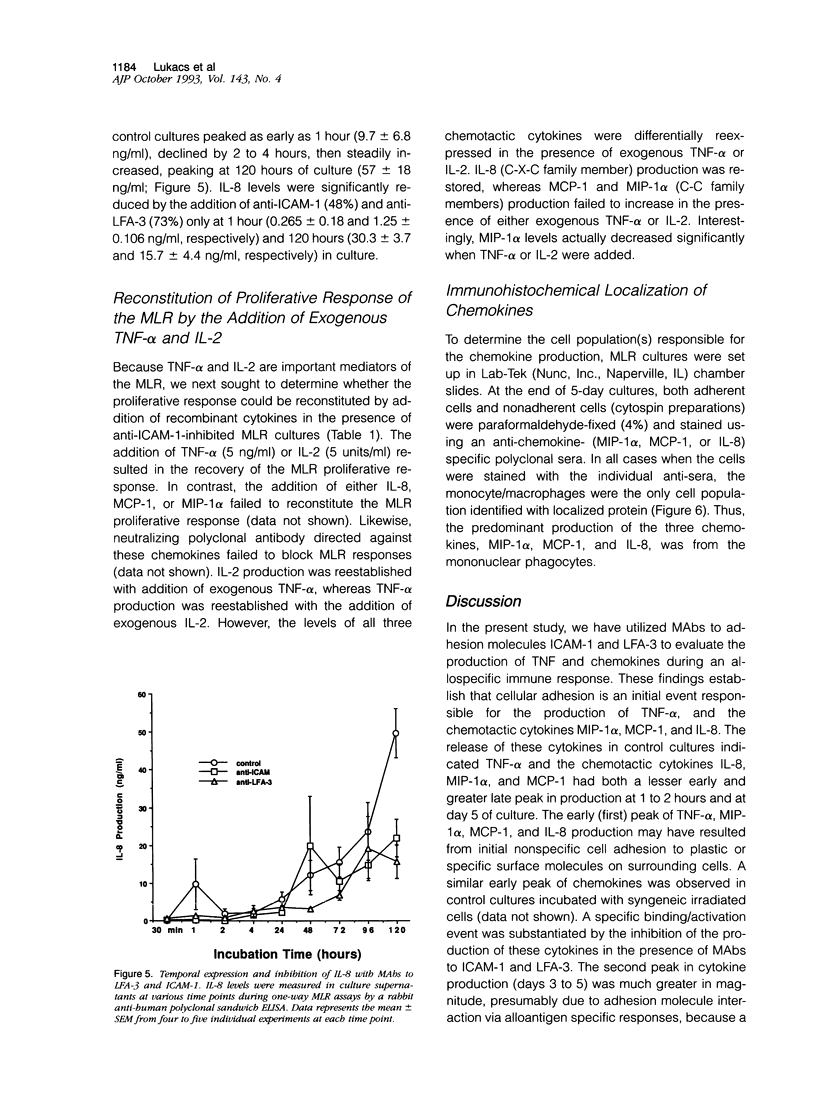
The in vitro mixed lymphocyte reaction (MLR) is regarded as a model of responsiveness to allogeneic major histocompatibility complex antigens and has historically been used to elucidate the pathway of T lymphocyte proliferation. In addition, the MLR response may reflect activation pathways relevant in acute allograft rejection. In the present study, we have applied the MLR to examine the role of adhesion molecules intercellular adhesion molecule-1 and lymphocyte function-associated antigen-3 in the induction of tumor necrosis factor-alpha (TNF-alpha) as well as chemotactic cytokines, interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1 alpha (MIP-1 alpha). Monoclonal antibodies to the adhesion molecules (5 micrograms/ml) were added to one-way human MLR cultures and supernatants collected at various time points. The monoclonal antibodies to the adhesion molecules significantly suppressed the proliferative response by 50 to 80%. Cytokine production, TNF-alpha (3.2 +/- 0.5 ng/ml), MIP-1 alpha (12.9 +/- 3.3 ng/ml), MCP-1 (18.8 +/- 3.4 ng/ml), and IL-8 (57 +/- 18 ng/ml) peaked on day 5 of the assay. The addition of anti-intercellular adhesion molecule-1 to the cultures suppressed TNF-alpha, MIP-1 alpha, MCP-1, and IL-8 production by 68% (1.05 +/- 0.29 ng/ml), 85% (2.0 +/- 1.2 ng/ml), 63% (6.8 +/- 2.9 ng/ml), and 47% (30.3 +/- 3.7 ng/ml), respectively. Likewise, the addition of anti-lymphocyte function-associated antigen-3 monoclonal antibody suppressed the cytokines by 78% (0.71 +/- 0.34 ng/ml), 66% (4.5 +/- 2.2 ng/ml), 52% (8.8 +/- 2.2 ng/ml), and 73% (15.7 +/- 4.4 ng/ml), respectively. Immunohistochemical staining indicated that monocytes were the primary source of the chemokines IL-8, MCP-1, and MIP-1 alpha. The addition of exogenous recombinant TNF-alpha (5 ng/ml) or recombinant IL-2 (5 units/ml) to the anti-intercellular adhesion molecule-1-treated cultures allowed the recovery of the proliferative response as well as restoration of IL-2, TNF-alpha, and IL-8, but not MCP-1 or MIP-1 alpha, indicating that both soluble and adhesion molecule signals are required for the production of the C-C family of chemokines in allogeneic responses. Thus, the events resulting in cellular proliferation and chemokine production were dependent on adhesion molecule interactions.
Full text
PDF


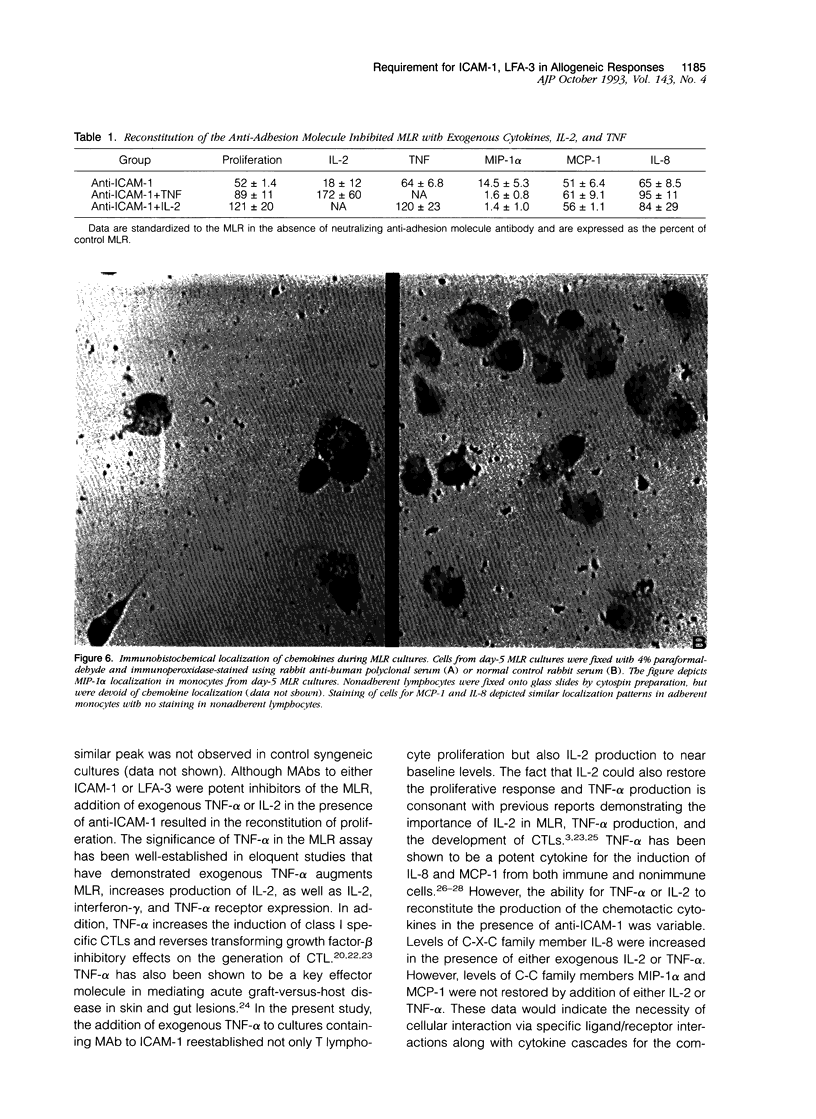



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco M., Pesce G., Prozato C., Canonica G. W. Functional involvement of the LFA-1/ICAM-1 adhesion system in the autologous mixed lymphocyte reaction. Cell Immunol. 1990 Jul;128(2):362–369. doi: 10.1016/0008-8749(90)90033-n. [DOI] [PubMed] [Google Scholar]
- Brooks B., Parry H., Lawry J., Rees R. Evidence that interleukin-4 suppression of lymphokine-activated killer cell induction is mediated through monocytes. Immunology. 1992 Feb;75(2):343–348. [PMC free article] [PubMed] [Google Scholar]
- Burton J., Goldman C. K., Rao P., Moos M., Waldmann T. A. Association of intercellular adhesion molecule 1 with the multichain high-affinity interleukin 2 receptor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7329–7333. doi: 10.1073/pnas.87.18.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosimi A. B., Conti D., Delmonico F. L., Preffer F. I., Wee S. L., Rothlein R., Faanes R., Colvin R. B. In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J Immunol. 1990 Jun 15;144(12):4604–4612. [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992 Apr 1;148(7):1985–1992. [PubMed] [Google Scholar]
- Deckert M., Kubar J., Bernard A. CD58 and CD59 molecules exhibit potentializing effects in T cell adhesion and activation. J Immunol. 1992 Feb 1;148(3):672–677. [PubMed] [Google Scholar]
- Dustin M. L., Carpen O., Springer T. A. Regulation of locomotion and cell-cell contact area by the LFA-1 and ICAM-1 adhesion receptors. J Immunol. 1992 May 1;148(9):2654–2663. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Chain B. M., Davies D. H., Ibrahim M. A., Katz D. R., Kaye P. M., Lightstone E. Antigen presentation by dendritic cells provides optimal stimulation for the production of interleukin (IL) 2, IL 4 and interferon-gamma by allogeneic T cells. Eur J Immunol. 1991 Nov;21(11):2803–2809. doi: 10.1002/eji.1830211123. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Pavilack M. A., Elner S. G., Remick D. G., Danforth J. M., Kunkel S. L. Human corneal interleukin-8. IL-1 and TNF-induced gene expression and secretion. Am J Pathol. 1991 Nov;139(5):977–988. [PMC free article] [PubMed] [Google Scholar]
- Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Tracey K. J., Tekamp-Olson P., Cousens L. S., Jones W. G., Shires G. T., Cerami A., Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992 May 1;148(9):2764–2769. [PubMed] [Google Scholar]
- Geissler D., Gaggl S., Möst J., Greil R., Herold M., Dietrich M. A monoclonal antibody directed against the human intercellular adhesion molecule (ICAM-1) modulates the release of tumor necrosis factor-alpha, interferon-gamma and interleukin 1. Eur J Immunol. 1990 Dec;20(12):2591–2596. doi: 10.1002/eji.1830201210. [DOI] [PubMed] [Google Scholar]
- Hirokawa M., Takatsu H., Ohshima A., Chubachi A., Kudo K., Niitsu H., Takahashi T., Yoshida K., Miura A. B. Lymphokine activity production in graft-versus-host reactions across minor histocompatibility antigen barriers. Clin Exp Immunol. 1989 Sep;77(3):434–439. [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Jin B. Q., Lopez A. F., Gillis S., Juttner C. A., Vadas M. A., Burns G. F. Human interleukin 4 regulates the phenotype of lymphocytes generated during mixed lymphocyte culture and inhibits the IL-2-induced development of LAK function in normal and leukaemic cells. Leuk Res. 1989;13(4):297–305. doi: 10.1016/0145-2126(89)90066-0. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Damle N. K., Blake J., Aruffo A., Ledbetter J. A. CD2/LFA-3 ligation induces phospholipase-C gamma 1 tyrosine phosphorylation and regulates CD3 signaling. J Immunol. 1992 Apr 1;148(7):2023–2029. [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Zachariae C. O., Oppenheim J. J., Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1403–1408. doi: 10.1016/s0006-291x(89)80160-3. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Lombardi G., Batchelor J. R., Reinsmoen N., Bach F. H. The molecular basis of alloreactivity. Immunol Today. 1990 Mar;11(3):83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today. 1990 Mar;11(3):97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Takahashi K., Fukazawa T., Koyanagi M., Yokoyama A., Kato H., Yagita H., Okumura K. Relative contribution of CD2 and LFA-1 to murine T and natural killer cell functions. J Immunol. 1990 Dec 1;145(11):3628–3634. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinet E., Branellec D., Termijtelen A. M., Blay J. Y., Gay F., Chouaib S. Evidence for tumor necrosis factor-alpha involvement in the optimal induction of class I allospecific cytotoxic T cells. J Immunol. 1990 Jun 15;144(12):4555–4561. [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Shalaby M. R., Espevik T., Rice G. C., Ammann A. J., Figari I. S., Ranges G. E., Palladino M. A., Jr The involvement of human tumor necrosis factors-alpha and -beta in the mixed lymphocyte reaction. J Immunol. 1988 Jul 15;141(2):499–503. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Lynch J. P., 3rd, Spengler R. N., Kunkel S. L. Interleukin-2-induced tumor necrosis factor-alpha (TNF-alpha) gene expression in human alveolar macrophages and blood monocytes. Am Rev Respir Dis. 1989 Feb;139(2):335–342. doi: 10.1164/ajrccm/139.2.335. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Wiggins R., Phan S. H., Wharram B. L., Showell H. J., Remick D. G., Chensue S. W., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- van Seventer G. A., Newman W., Shimizu Y., Nutman T. B., Tanaka Y., Horgan K. J., Gopal T. V., Ennis E., O'Sullivan D., Grey H. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991 Oct 1;174(4):901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]