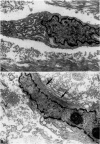
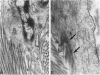
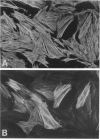
Abstract
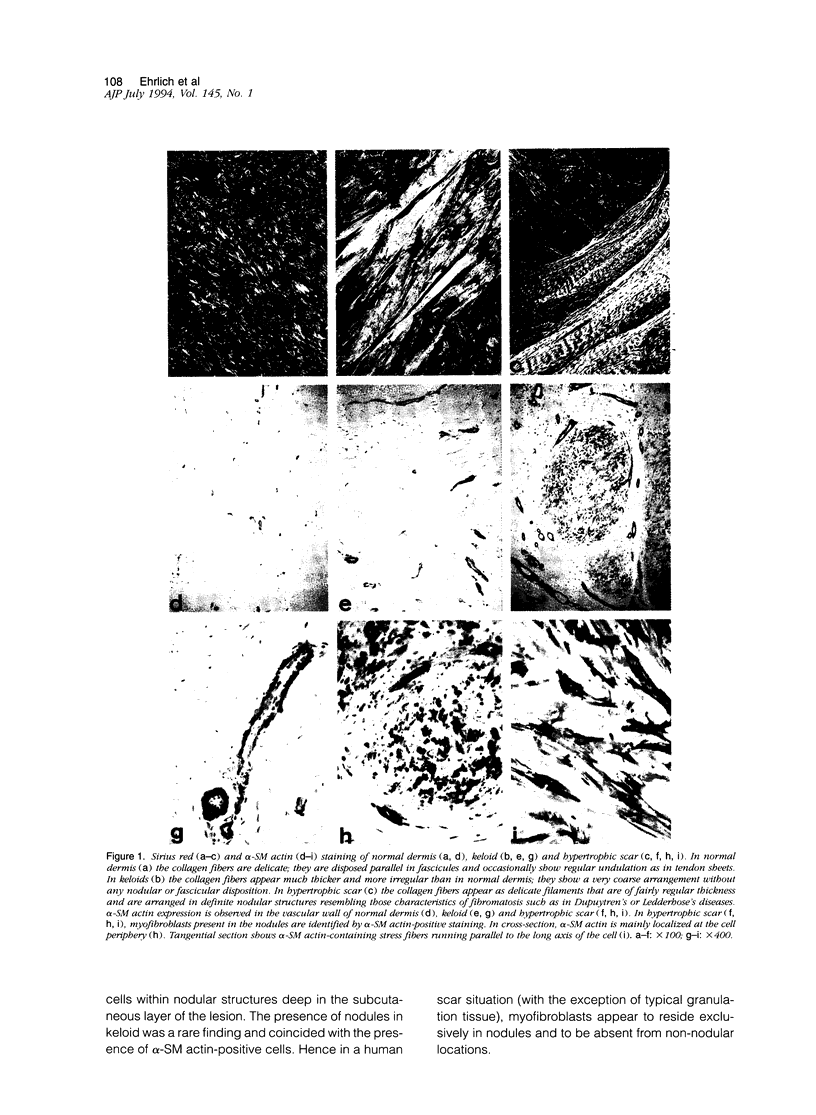
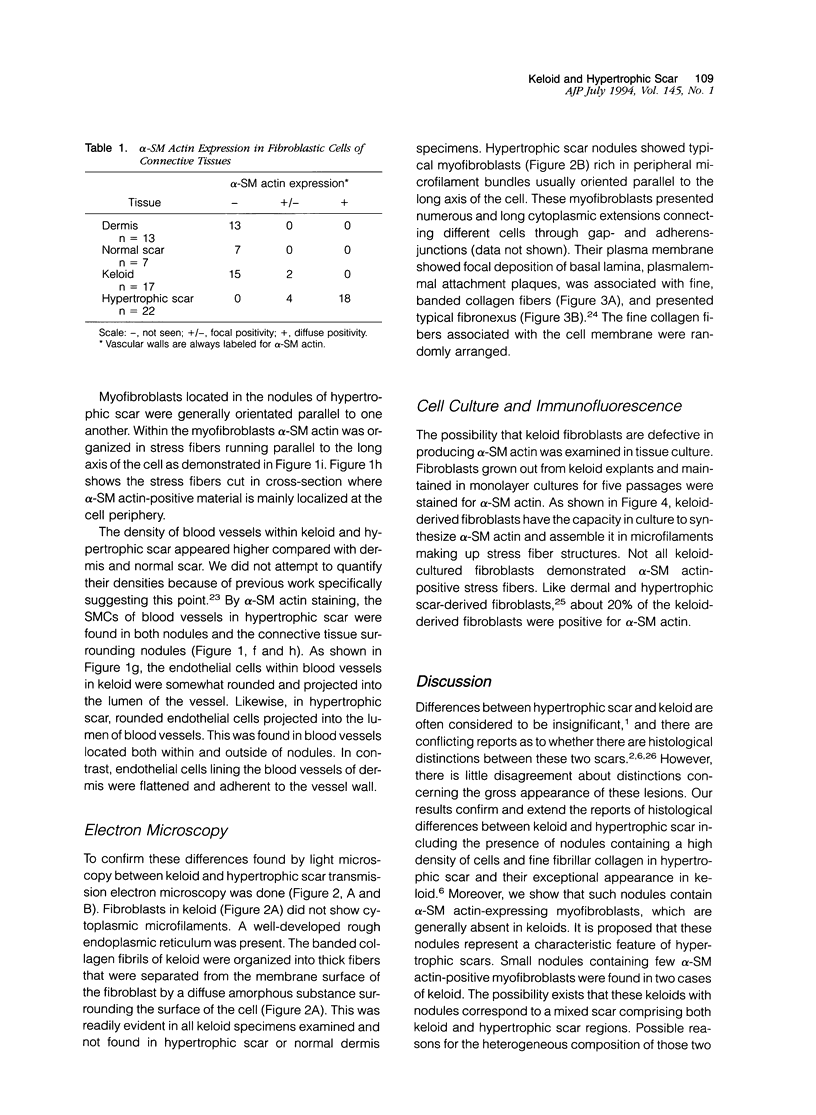
There are two types of excessive scarring, keloid and hypertrophic scar. Contrary to hypertrophic scars, keloids do not regress with time, are difficult to revise surgically, and do not provoke scar contractures. These two lesions require different therapeutic approaches but are often confused because of an apparent lack of morphological differences. We have investigated the collagen organization and the possible presence of alpha-smooth muscle (SM) actin-expressing myofibroblasts in these conditions. Keloids contain large, thick collagen fibers composed of numerous fibrils closely packed together. In contrast hypertrophic scars exhibit modular structures in which fibroblastic cells, small vessels, and fine, randomly organized collagen fibers are present. We confirm that such nodular structures are always present in hypertrophic scar and rarely in keloid. Furthermore, only nodules of hypertrophic scars contain alpha-SM actin-expressing myofibroblasts. Electron microscopic examination supports the above-mentioned differences in collagen organization and in fibroblastic features and shows the presence of an amorphous extracellular material surrounding fibroblastic cells in keloid. The presence in hypertrophic scar myofibroblasts of alpha-SM actin, the actin isoform typical of vascular SM cells, may represent an important element in the pathogenesis of contraction. Interestingly, when placed in culture fibroblasts from hypertrophic scars and keloid express similar amounts of alpha-SM actin, suggesting that local microenvironmental factors influence in vivo the expression of this protein. Thus several morphological and immunohistochemical differences exist between hypertrophic scar and keloid that are useful for the biological and pathological characterization of the two lesions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew J. G., Andrew S. M., Ash A., Turner B. An investigation into the role of inflammatory cells in Dupuytren's disease. J Hand Surg Br. 1991 Aug;16(3):267–271. doi: 10.1016/0266-7681(91)90051-o. [DOI] [PubMed] [Google Scholar]
- Baur P. S., Larson D. L., Stacey T. R. The observation of myofibroblasts in hypertrophic scars. Surg Gynecol Obstet. 1975 Jul;141(1):22–26. [PubMed] [Google Scholar]
- Blackburn W. R., Cosman B. Histologic basis of keloid and hypertrophic scar differentiation. Clinicopathologic correlation. Arch Pathol. 1966 Jul;82(1):65–71. [PubMed] [Google Scholar]
- Constantine V. S., Mowry R. W. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968 May;50(5):419–423. doi: 10.1038/jid.1968.68. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Abdiu A., Walz T., Macieira-Coelho A., Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992 Jul;201(1):64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Eddy R. J., Petro J. A., Tomasek J. J. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- GLUCKSMANN A. Local factors in the histogenesis of hypertrophic scars. Br J Plast Surg. 1951 Jul;4(2):88–103. doi: 10.1016/s0007-1226(51)80014-6. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Majno G. Dupuytren's contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972 Jan;66(1):131–146. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994 Feb;124(4):401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembry R. M., Bernanke D. H., Hayashi K., Trelstad R. L., Ehrlich H. P. Morphologic examination of mesenchymal cells in healing wounds of normal and tight skin mice. Am J Pathol. 1986 Oct;125(1):81–89. [PMC free article] [PubMed] [Google Scholar]
- Kischer C. W., Brody G. S. Structure of the collagen nodule from hypertrophic scars and keloids. Scan Electron Microsc. 1981;(Pt 3):371–376. [PubMed] [Google Scholar]
- Kischer C. W. Fibroblasts of the hypertrophic scar, mature scar and normal skin: A study by scanning and transmission electron microscopy. Tex Rep Biol Med. 1974 Fall-Winter;32(3-4):699–709. [PubMed] [Google Scholar]
- Kischer C. W., Shetlar M. R., Chvapil M. Hypertrophic scars and keloids: a review and new concept concerning their origin. Scan Electron Microsc. 1982;(Pt 4):1699–1713. [PubMed] [Google Scholar]
- Kischer C. W., Thies A. C., Chvapil M. Perivascular myofibroblasts and microvascular occlusion in hypertrophic scars and keloids. Hum Pathol. 1982 Sep;13(9):819–824. doi: 10.1016/s0046-8177(82)80078-6. [DOI] [PubMed] [Google Scholar]
- Lametschwandtner A., Staindl O. Zur Angioarchitektur des Keloids. Eine rasterelektronenmikroskopische Untersuchung an Korrosionspräparaten. HNO. 1990 Jun;38(6):202–207. [PubMed] [Google Scholar]
- Lawrence W. T. In search of the optimal treatment of keloids: report of a series and a review of the literature. Ann Plast Surg. 1991 Aug;27(2):164–178. doi: 10.1097/00000637-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Linares H. A., Kischer C. W., Dobrkovsky M., Larson D. L. The histiotypic organization of the hypertrophic scar in humans. J Invest Dermatol. 1972 Oct;59(4):323–331. doi: 10.1111/1523-1747.ep12627386. [DOI] [PubMed] [Google Scholar]
- MANCINI R. E., QUAIFE J. V. Histogenesis of experimentally produced keloids. J Invest Dermatol. 1962 Mar;38:143–181. doi: 10.1038/jid.1962.29. [DOI] [PubMed] [Google Scholar]
- Peacock E. E., Jr, Madden J. W., Trier W. C. Biologic basis for the treatment of keloids and hypertrophic scars. South Med J. 1970 Jul;63(7):755–760. doi: 10.1097/00007611-197007000-00002. [DOI] [PubMed] [Google Scholar]
- Rockwell W. B., Cohen I. K., Ehrlich H. P. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989 Nov;84(5):827–837. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Kazazis D. M., Clark R. A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984 Jun;98(6):2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Welch M. P., Odland G. F., Clark R. A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990 Jan;110(1):133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]