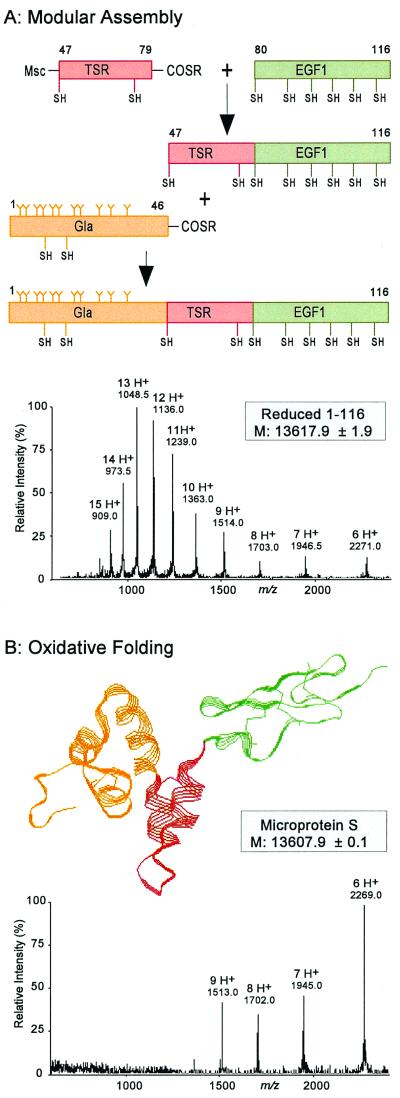
Figure 2.
Chemical synthesis of microprotein S by modular synthesis and multistep native chemical ligation. (A) Synthetic scheme leading to the 116-residue microprotein S polypeptide chain. To avoid polymerization or cycliza- tion reactions of the middle peptide segment corresponding to the TSR (residues 47–79), the N-terminal Cys residue of the TSR peptide-thioester was reversibly protected with an Msc group (24, 27). After ligation to the EGF1 module peptide (residues 80–116) was completed, the N-terminal Msc was removed, and the Gla-rich peptide (residues 1–46) was ligated to the TSR-EGF1 polypeptide (residues 47–116). ESI-MS of the reduced 1–116 polypeptide chain of microprotein S revealed a mass of 13,618 Da, corresponding to the theoretical mass of microprotein S containing 11 Gla residues in the Gla module. (B) After folding of the 1–116 polypeptide in 1 M guanidine at pH 8.0, microprotein S was obtained with an apparent molecular mass of 13,608 Da, the decrease of 10 Da caused by loss of 10 protons as a result of the formation of 5 disulfide bonds. Note the change in charged state distribution of the folded microprotein S to higher m/z ratios, which is characteristic behavior of folded proteins. The three-dimensional microprotein S structure shown is based on molecular homology modeling (28).