Abstract
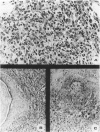
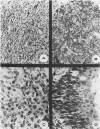
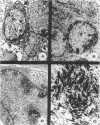
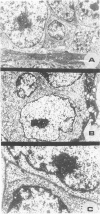
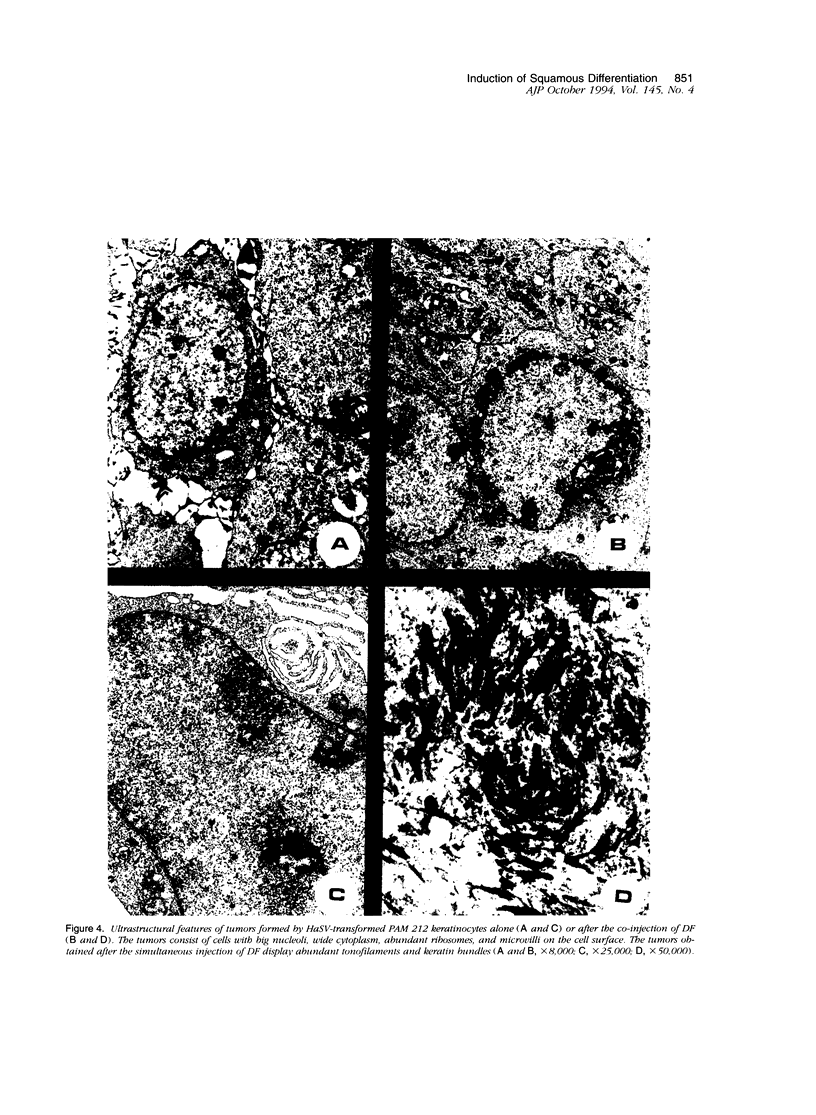
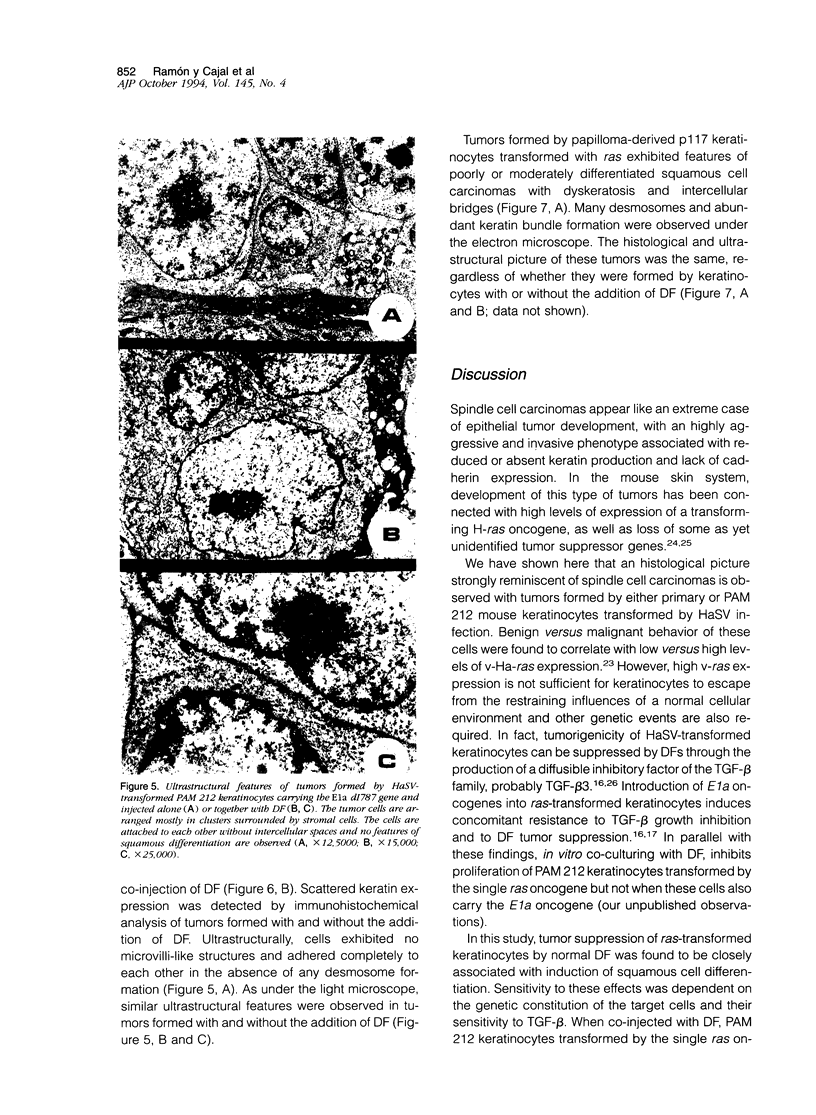
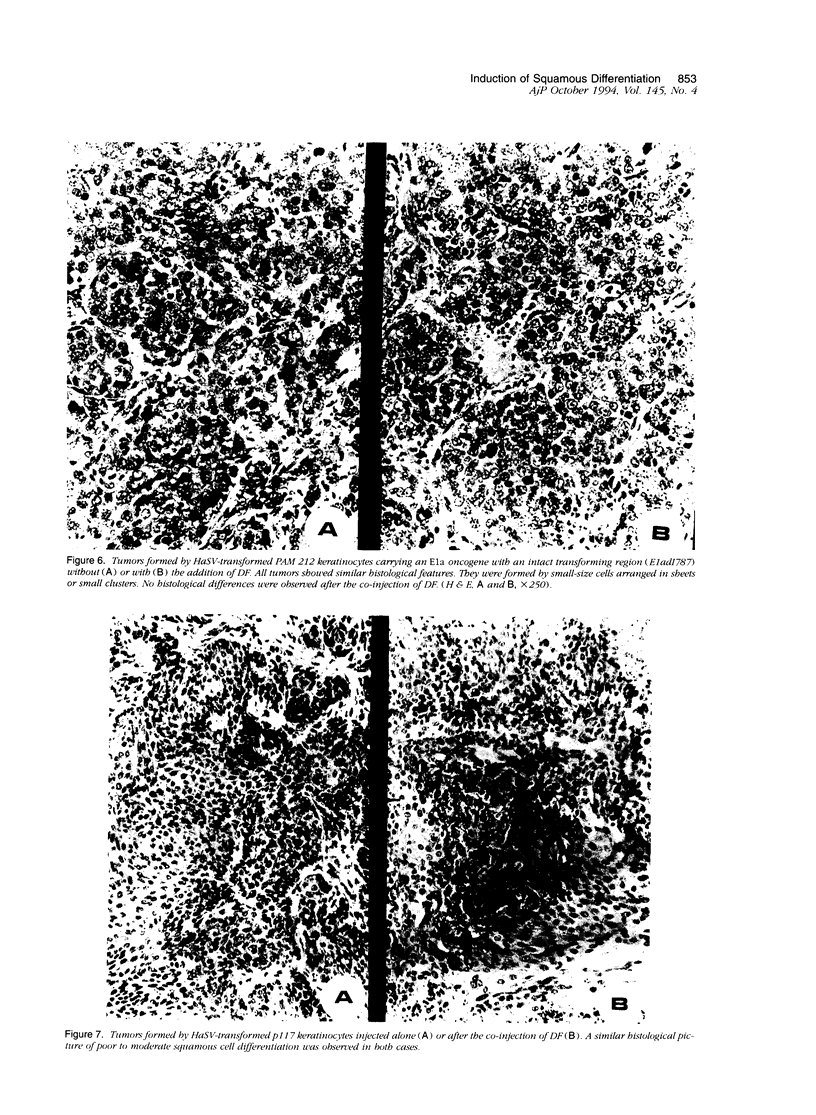
We have previously reported that tumor formation of ras-transformed keratinocytes can be suppressed by dermal fibroblasts through production of a diffusible growth inhibitory factor of the transforming growth factor-beta (TGF-beta) family. Keratinocytes transformed by ras and E1a oncogenes or papilloma-derived keratinocytes transformed by a ras oncogene show concomitant resistance to dermal fibroblast tumor suppression and TGF-beta growth inhibition. We report here that dermal fibroblast tumor suppression is associated with a striking induction of squamous cell differentiation and that this effect is blocked in tumors resistant to dermal fibroblast inhibition. This experimental system strongly supports the notion that suppression of tumorigenicity and induction of a differentiated phenotype are closely associated events.
Full text
PDF
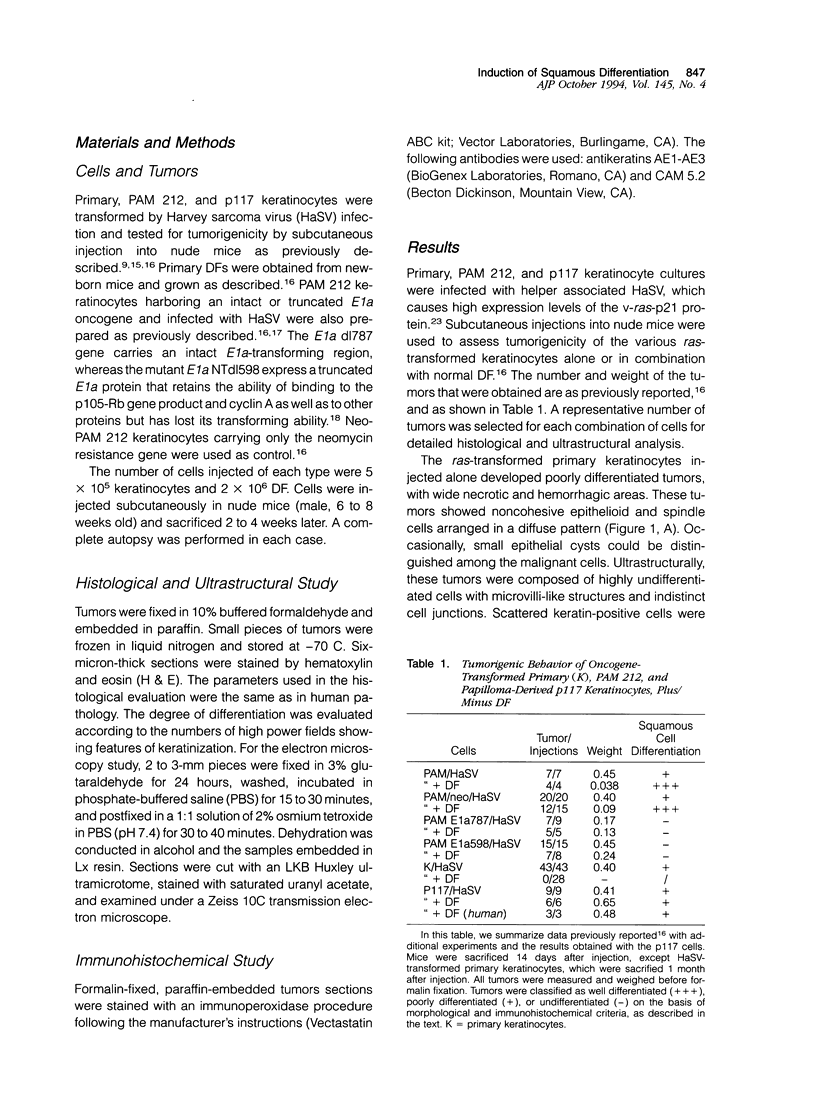

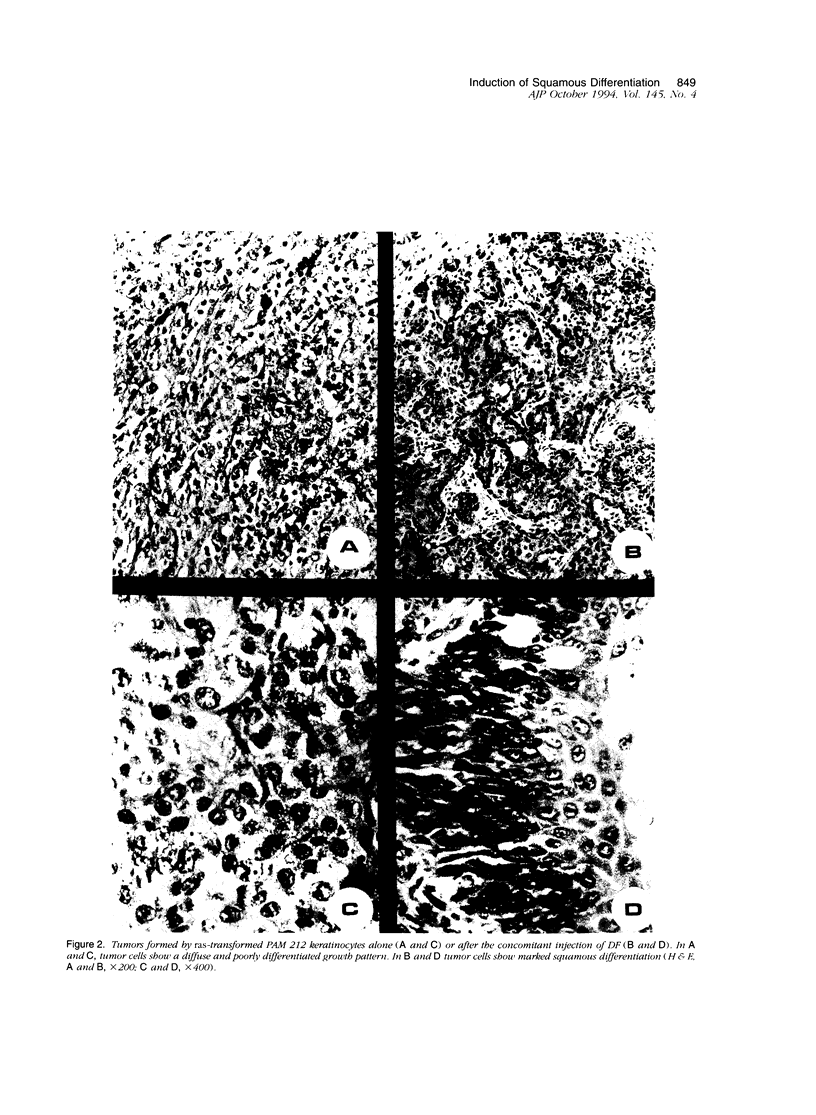
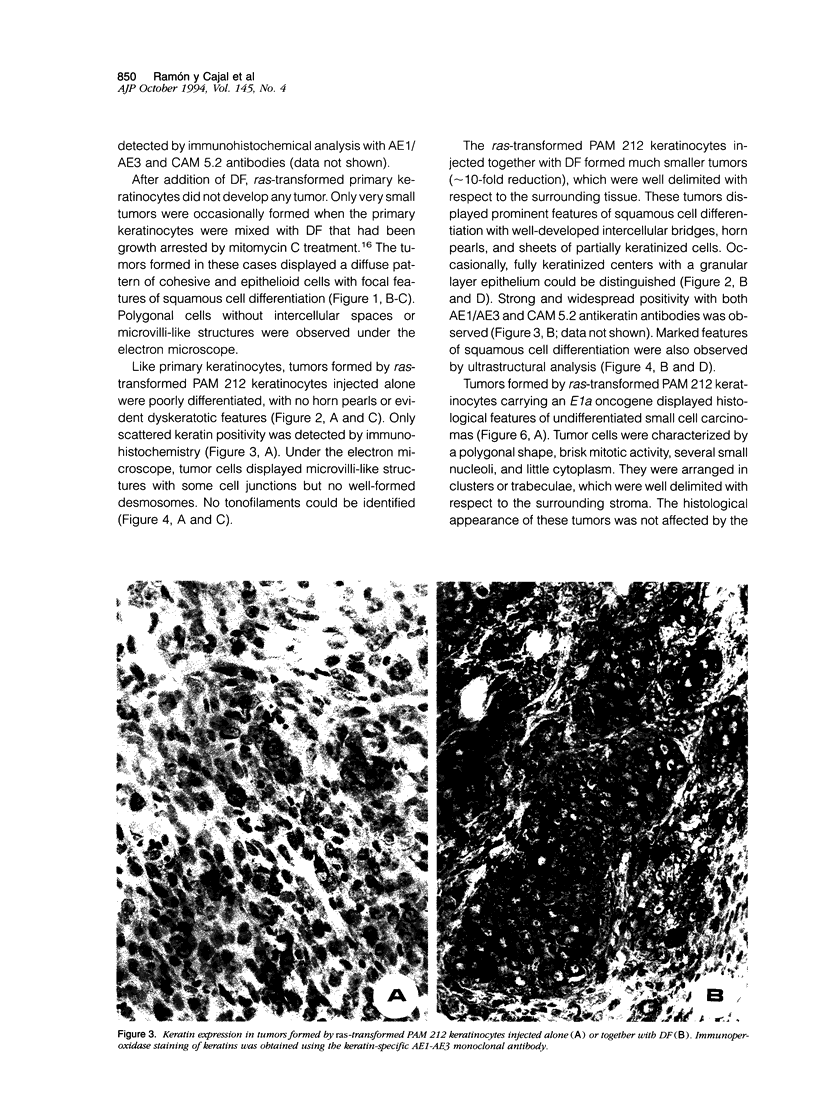


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette J. L., Missero C., Yuspa S. H., Dotto G. P. Different levels of v-Ha-ras p21 expression in primary keratinocytes transformed with Harvey sarcoma virus correlate with benign versus malignant behavior. Mol Carcinog. 1993;7(1):21–25. doi: 10.1002/mc.2940070105. [DOI] [PubMed] [Google Scholar]
- Buchmann A., Ruggeri B., Klein-Szanto A. J., Balmain A. Progression of squamous carcinoma cells to spindle carcinomas of mouse skin is associated with an imbalance of H-ras alleles on chromosome 7. Cancer Res. 1991 Aug 1;51(15):4097–4101. [PubMed] [Google Scholar]
- Busch H. The final common pathway of cancer. Cancer Res. 1990 Aug 15;50(16):4830–4838. [PubMed] [Google Scholar]
- Dexter D. L., Calabresi P. Intraneoplastic diversity. Biochim Biophys Acta. 1982 Dec 21;695(2):97–112. doi: 10.1016/0304-419x(82)90019-1. [DOI] [PubMed] [Google Scholar]
- Dexter D. L., Spremulli E. N., Fligiel Z., Barbosa J. A., Vogel R., VanVoorhees A., Calabresi P. Heterogeneity of cancer cells from a single human colon carcinoma. Am J Med. 1981 Dec;71(6):949–956. doi: 10.1016/0002-9343(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., O'Connell J., Patskan G., Conti C., Ariza A., Slaga T. J. Malignant progression of papilloma-derived keratinocytes: differential effects of the ras, neu, and p53 oncogenes. Mol Carcinog. 1988;1(3):171–179. doi: 10.1002/mc.2940010305. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Weinberg R. A., Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6389–6393. doi: 10.1073/pnas.85.17.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escherick J. S., DiCunto F., Flanders K. C., Missero C., Dotto G. P. Transforming growth factor beta 1 induction is associated with transforming growth factors beta 2 and beta 3 down-modulation in 12-O-tetradecanoylphorbol-13-acetate-induced skin hyperplasia. Cancer Res. 1993 Nov 15;53(22):5517–5522. [PubMed] [Google Scholar]
- Florin-Christensen M., Missero C., Dotto G. P., Florin-Christensen J. The E1a gene prevents inhibition of keratinocyte proliferation by dexamethasone. Exp Cell Res. 1992 Nov;203(1):285–288. doi: 10.1016/0014-4827(92)90067-i. [DOI] [PubMed] [Google Scholar]
- Florin-Christensen M., Missero C., Florin-Christensen J., Tranque P., Ramon y Cajal S., Dotto G. P. Counteracting effects of E1a transformation on cAMP growth inhibition. Exp Cell Res. 1993 Jul;207(1):57–61. doi: 10.1006/excr.1993.1162. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Kulkarni A. B., Tennenbaum T., Hennings H., Flanders K. C., O'Reilly M., Sporn M. B., Karlsson S., Yuspa S. H. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6076–6080. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Heppner G. H. Tumor heterogeneity. Cancer Res. 1984 Jun;44(6):2259–2265. [PubMed] [Google Scholar]
- Luikart S. D. Effects of extracellular matrix on the malignant phenotype. Yale J Biol Med. 1988 Jan-Feb;61(1):35–38. [PMC free article] [PubMed] [Google Scholar]
- Missero C., Filvaroff E., Dotto G. P. Induction of transforming growth factor beta 1 resistance by the E1A oncogene requires binding to a specific set of cellular proteins. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3489–3493. doi: 10.1073/pnas.88.8.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. E., Tarin D., Fidler I. J. Influence of organ microenvironment on pigmentation of a metastatic murine melanoma. Cancer Res. 1988 Apr 15;48(8):2258–2264. [PubMed] [Google Scholar]
- Ramon y Cajal S., Suster S., Halaban R., Filvaroff E., Dotto G. P. Induction of different morphologic features of malignant melanoma and pigmented lesions after transformation of murine melanocytes with bFGF-cDNA and H-ras, myc, neu, and E1a oncogenes. Am J Pathol. 1991 Feb;138(2):349–358. [PMC free article] [PubMed] [Google Scholar]
- Reiss M., Sartorelli A. C. Regulation of growth and differentiation of human keratinocytes by type beta transforming growth factor and epidermal growth factor. Cancer Res. 1987 Dec 15;47(24 Pt 1):6705–6709. [PubMed] [Google Scholar]
- Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985 Jul;45(7):2935–2942. [PubMed] [Google Scholar]
- Sartorelli A. C. The 1985 Walter Hubert lecture. Malignant cell differentiation as a potential therapeutic approach. Br J Cancer. 1985 Sep;52(3):293–302. doi: 10.1038/bjc.1985.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland J. E., Greenhalgh D. A., Koceva-Chyla A., Hennings H., Restrepo C., Balaschak M., Yuspa S. H. Development of murine epidermal cell lines which contain an activated rasHa oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988 Jan 1;48(1):165–169. [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F. Cellular heterogeneity in tumours. Br J Cancer. 1983 May;47(5):589–594. doi: 10.1038/bjc.1983.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]