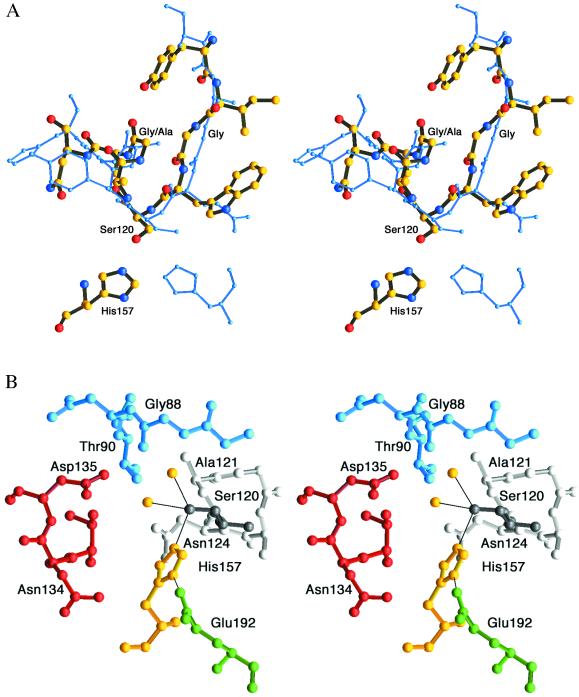
Figure 2.
(A) Strand–helix motif. Superimposition of aspartyl dipeptidase (thick multicolored model) and serine carboxypeptidase (blue) illustrates similarities of the strand–helix motifs and the different histidine positions (35). The Cα atoms of the nine residues surrounding the active site Ser were superimposed and have a rms deviation of 0.9 Å. (B) View of the active site with Ser120 (dark gray) and the helix following it with Ala121-Asn124 (light gray). The active site Ser is coordinated to His157 and two water molecules (yellow). Glu192 is shown in green, Thr133-Asp135 in red, and Gly87-Thr90 in blue.