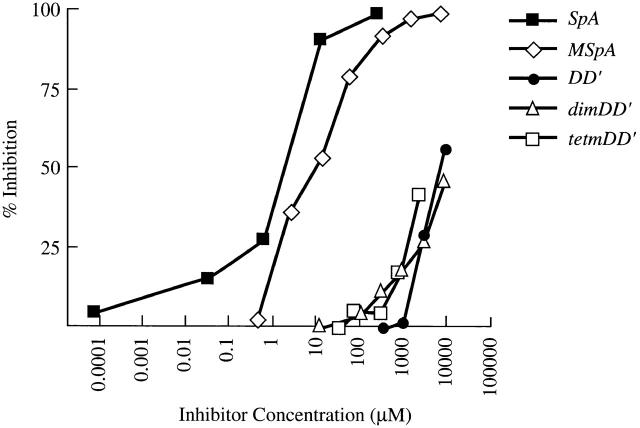
Figure 2.
Recombinant forms of SpA display a broad range of Fab-binding avidities. The relative Fab-binding activities of five different forms of SpA were compared in inhibition immunoassays of the binding of an S107-encoded IgG in solution to wells coated with an SpA variant. In these studies, the activity of native SpA is compared with a form of SpA created by chemical modification (MSpA), an SpA monomer, domain D (DD′), and dimeric (dimtetDD′) and tetrameric (tetmDD′) forms of domain D′, which include two site-specific mutations that remove Fc-binding activity.