Abstract
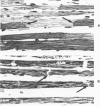
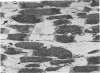
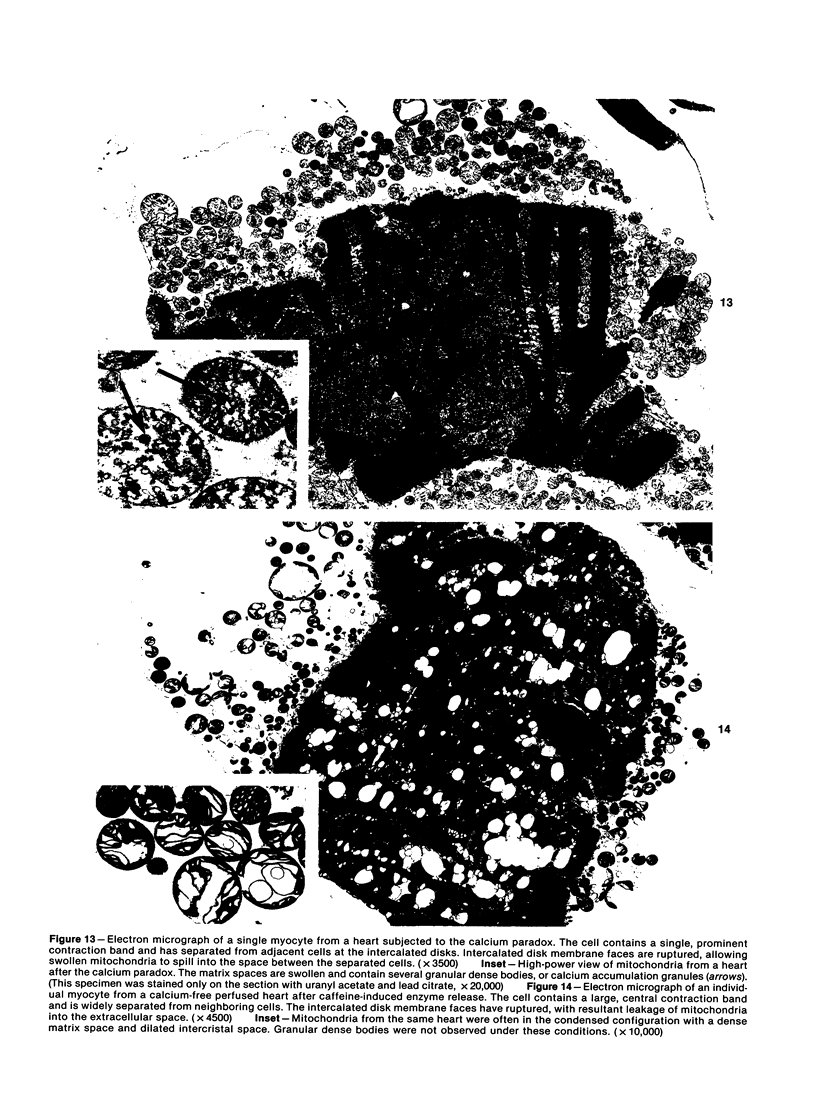
Hearts depleted of extracellular calcium become susceptible to injury caused by repletion of extracellular calcium (calcium paradox). It has been suggested that calcium-free perfusion causes weakening of intercalated disks and that the physical stress of contracture may cause sarcolemmal membrane rupture and creatine kinase (CK) release. To further investigate this hypothesis, the effects of caffeine on contracture, cellular morphology, and CK release were studied in control and calcium-free perfused isolated rat hearts. Control hearts perfused with 2.5 mM calcium retained normal ultrastructure for long periods of perfusion. Calcium-free hearts perfused for 12 minutes developed separations of fascia adherens portions of intercalated disks but retained intact nexus junctions. Hearts subjected to 5-minute calcium-free perfusion, followed by calcium repletion, developed a massive CK release and extensive contraction band necrosis (calcium paradox). Ten millimolar caffeine, which causes rapid calcium release from sarcoplasmic reticulum (SR), produced contracture, but not CK release, from control hearts perfused with medium containing 2.5 mM calcium. In calcium-free perfused hearts, caffeine caused sudden CK release accompanied by contracture, development of contraction bands, wide separations of cells at intercalated disks, and sarcolemmal membrane injury. Caffeine-induced injury occurred despite 3 mM amobarbital inhibition of mitochondrial respiration. Hearts perfused with caffeine in the presence of calcium relaxed when made calcium-free and did not release CK. Addition of caffeine following calcium-free perfusion at 22 C, which protects the heart from the calcium paradox, produced a rapid, transient contracture. These results are compatible with the hypothesis that myocardial cell injury in calcium-free hearts is not dependent on repletion of extracellular calcium or mitochondrial function, but can result from contracture following caffeine-induced release of intracellular calcium from the SR.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alto L. E., Dhalla N. S. Myocardial cation contents during induction of calcium paradox. Am J Physiol. 1979 Dec;237(6):H713–H719. doi: 10.1152/ajpheart.1979.237.6.H713. [DOI] [PubMed] [Google Scholar]
- Baker J. E., Bullock G. R., Hearse D. J. The temperature dependence of the calcium paradox: enzymatic, functional and morphological correlates of cellular injury. J Mol Cell Cardiol. 1983 Jun;15(6):393–411. doi: 10.1016/0022-2828(83)90323-1. [DOI] [PubMed] [Google Scholar]
- Blayney L., Thomas H., Muir J., Henderson A. Action of caffeine on calcium transport by isolated fractions of myofibrils, mitochondria, and sarcoplasmic reticulum from rabbit heart. Circ Res. 1978 Oct;43(4):520–526. doi: 10.1161/01.res.43.4.520. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Boink A. B., Ruigrok T. J., de Moes D., Maas A. H., Zimmerman A. N. The effect of hypothermia on the occurrence of the calcium paradox. Pflugers Arch. 1980 May;385(2):105–109. doi: 10.1007/BF00588688. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Das P. K., Sharma G. P. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol. 1978 Apr;10(4):363–385. doi: 10.1016/0022-2828(78)90384-x. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S. Involvement of membrane systems in heart failure due to intracellular calcium overload and deficiency. J Mol Cell Cardiol. 1976 Sep;08(9):661–667. doi: 10.1016/0022-2828(76)90008-0. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Langer G. A., Nudd L. M., Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its stucture and cellular ionic exchange. Circ Res. 1977 Nov;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Grinwald P. M., Nayler W. G. 2,4-Dinitrophenol (DNP)-induced injury in calcium-free hearts. J Mol Cell Cardiol. 1984 Jun;16(6):547–557. doi: 10.1016/s0022-2828(84)80641-0. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Liu S. Y., Safavi S., Kaltenbach J. P. Anoxia, calcium and contracture as mediators of myocardial enzyme release. J Mol Cell Cardiol. 1981 Jan;13(1):93–106. doi: 10.1016/0022-2828(81)90231-5. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Sims M. A. Parallel temperature dependence of contracture-associated enzyme release due to anoxia, 2,4-dinitrophenol (DNP), or caffeine and the calcium paradox. Am J Pathol. 1984 Jul;116(1):94–106. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., Sims M. A. Physical stress-mediated enzyme release from calcium-deficient hearts. J Mol Cell Cardiol. 1983 Jul;15(7):421–429. doi: 10.1016/0022-2828(83)90262-6. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Sims M. A., VanderHeide R. S. Mechanism of enzyme release in the calcium paradox. Eur Heart J. 1983 Dec;4 (Suppl H):63–71. doi: 10.1093/eurheartj/4.suppl_h.63. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Baker J. E., Humphrey S. M. Verapamil and the calcium paradox. J Mol Cell Cardiol. 1980 Jul;12(7):733–739. doi: 10.1016/0022-2828(80)90103-0. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Holland C. E., Jr, Olson R. E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975 Dec;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Cellular calcium turnover in the perfused rat heart: modulation by caffeine and procaine. Circ Res. 1982 Sep;51(3):363–370. doi: 10.1161/01.res.51.3.363. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A. Calcium physiology in smooth muscle. Prog Cardiovasc Dis. 1982 Nov-Dec;25(3):211–224. doi: 10.1016/0033-0620(82)90017-2. [DOI] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Grinwald P. M. The effect of verapamil on calcium accumulation during the calcium paradox. J Mol Cell Cardiol. 1981 Apr;13(4):435–441. doi: 10.1016/0022-2828(81)90285-6. [DOI] [PubMed] [Google Scholar]
- Pretorius P. J., Pohl W. G., Smithen C. S., Inesi G. Structural and functional characterization of dog heart microsomes. Circ Res. 1969 Oct;25(4):487–499. doi: 10.1161/01.res.25.4.487. [DOI] [PubMed] [Google Scholar]
- Rich T. L., Langer G. A. Calcium depletion in rabbit myocardium. Calcium paradox protection by hypothermia and cation substitution. Circ Res. 1982 Aug;51(2):131–141. doi: 10.1161/01.res.51.2.131. [DOI] [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Slade A., Zimmerman A. N., Meijler F. L., Nayler W. G. The effect of verapamil on the calcium paradox. Am J Pathol. 1980 Mar;98(3):769–790. [PMC free article] [PubMed] [Google Scholar]
- Ruigrok T. J., Boink A. B., Spies F., Blok F. J., Maas A. H., Zimmerman A. N. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978 Nov;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca2+-free medium. J Mol Cell Cardiol. 1975 Feb;7(2):91–103. doi: 10.1016/0022-2828(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]