Abstract
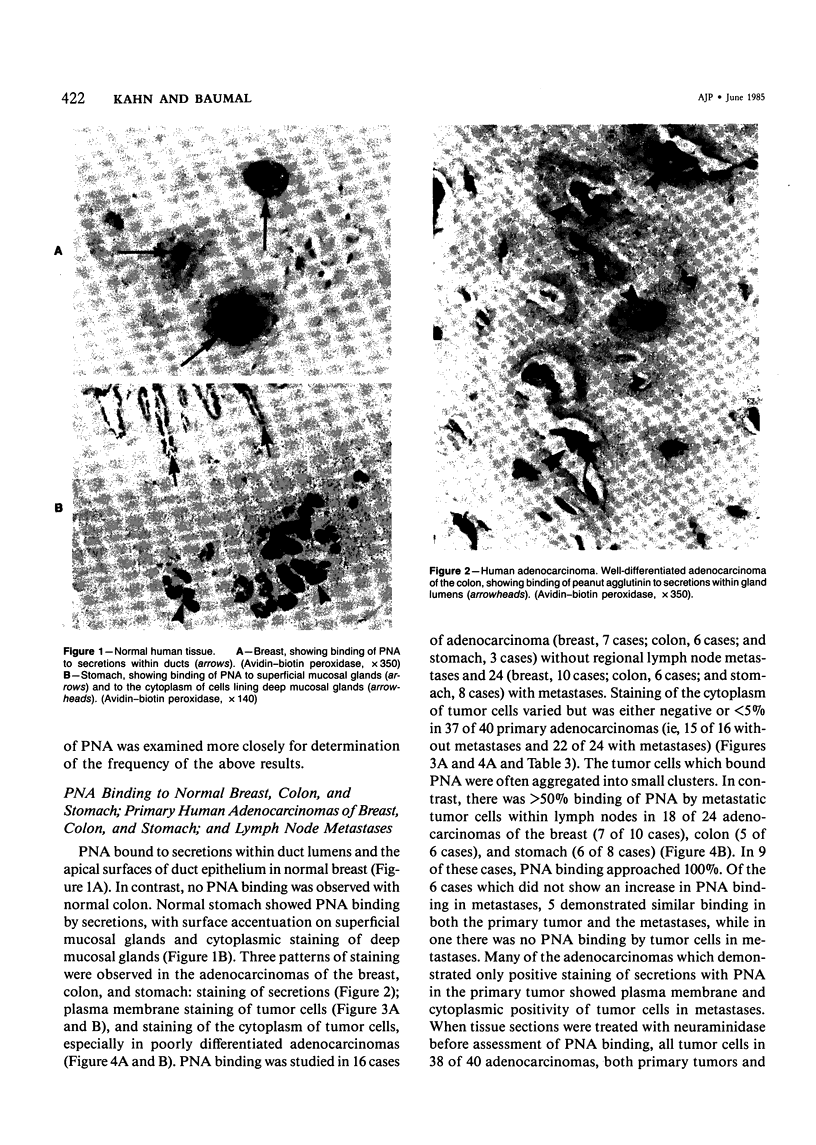
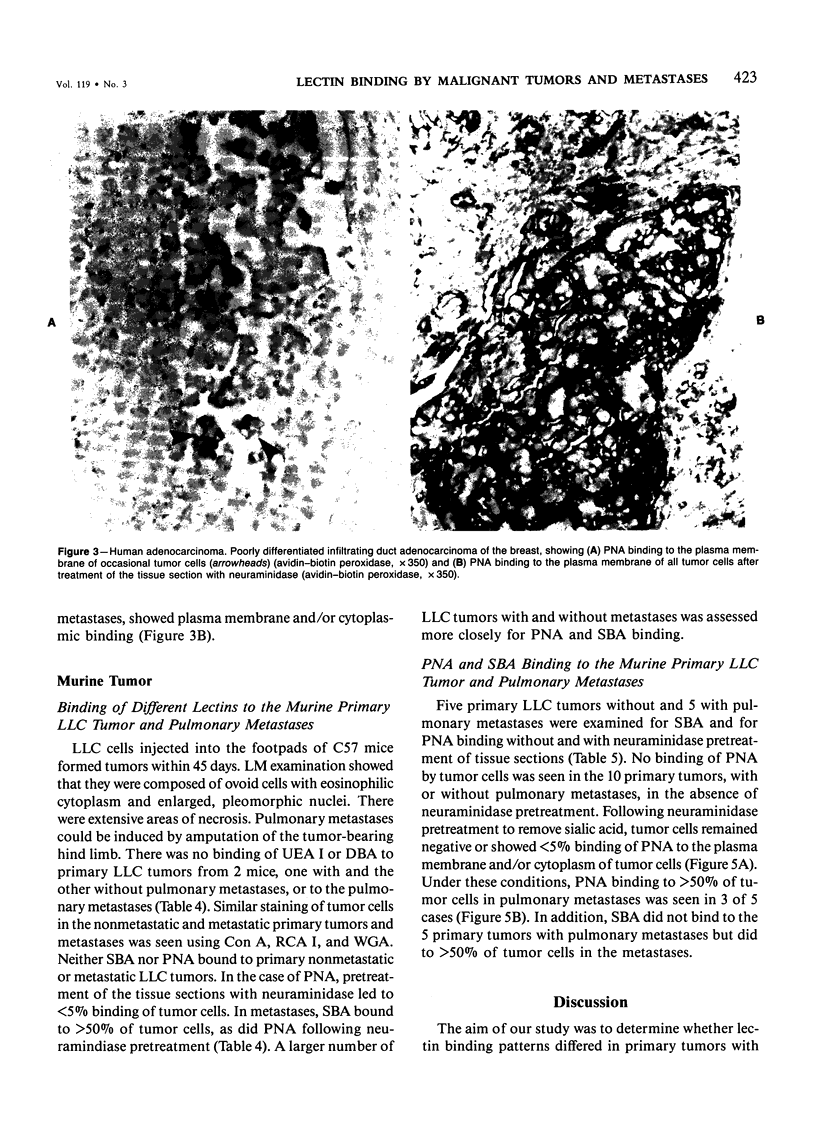
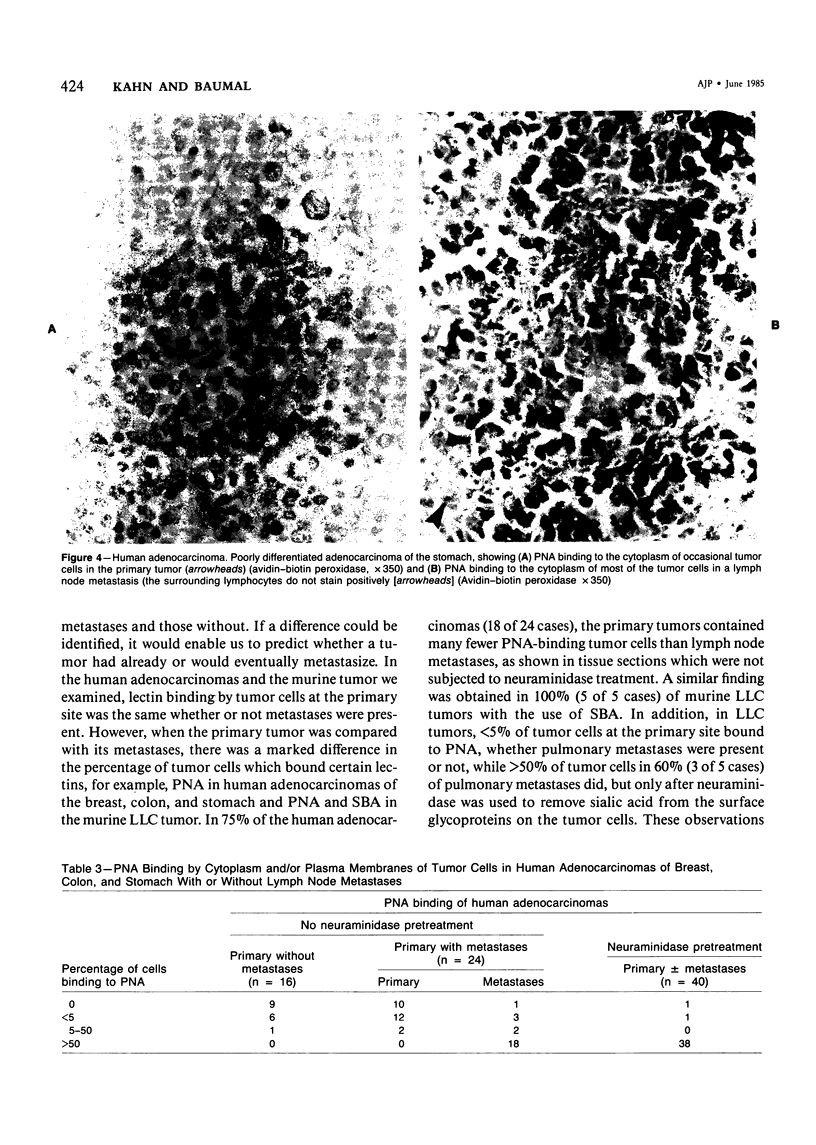
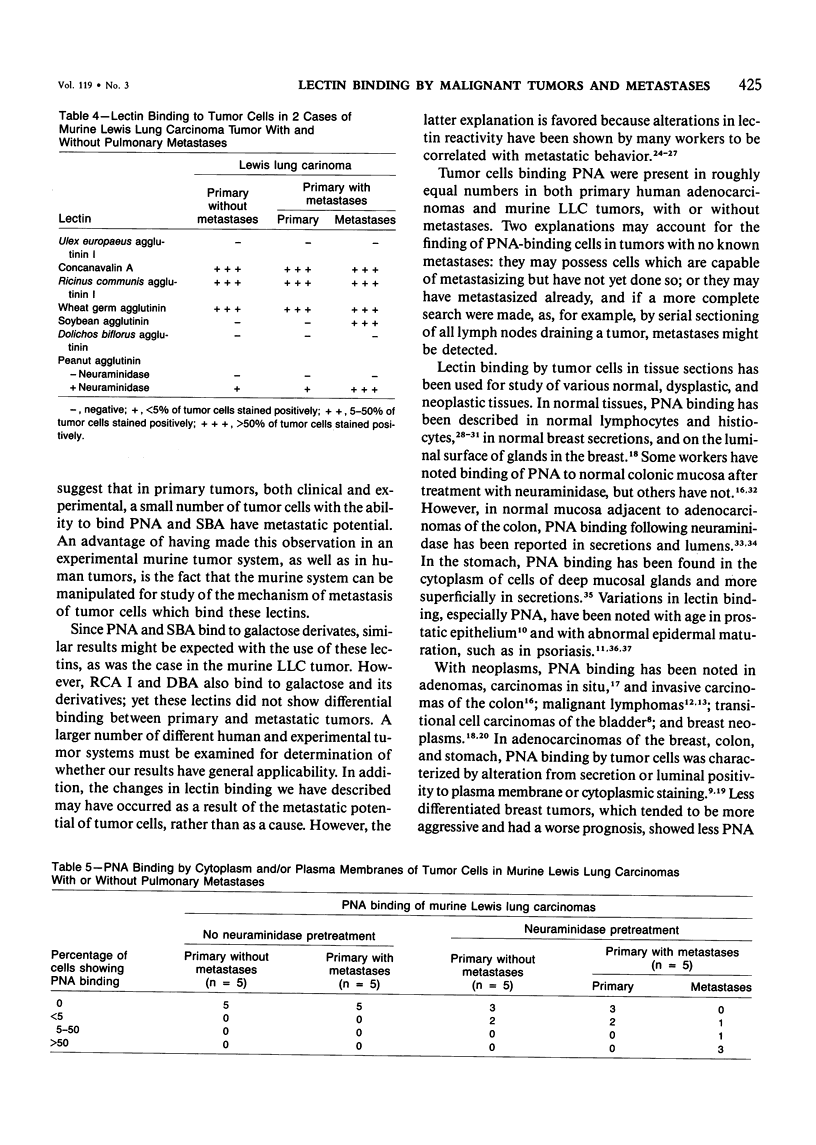
Lectin binding to tumor cells in tissue sections of 16 nonmetastatic and 24 metastatic human adenocarcinomas and 5 nonmetastatic and 5 metastatic murine Lewis lung carcinomas (LLCs) was assessed with an avidin-biotin peroxidase technique. In human tumors, Ulex europaeus agglutinin I (UEA I) showed no binding; whereas concanavalin A (Con A), Ricinus communis agglutinin I (RCA I), wheat germ agglutinin (WGA), soybean agglutinin (SBA), and Dolichos biflorus agglutinin (DBA) bound equally to primaries and metastases. However, peanut agglutinin (PNA) bound to less than 5% of cells in 37 of 40 primaries but to greater than 50% of cells in 18 of 24 metastases. In LLC tumors, UEA I and DBA showed no binding; whereas Con A, RCA I, and WGA bound equally to primaries and metastases. SBA bound to greater than 50% of cells in 5 metastases but not to the 5 primaries. There was less than 5% binding of PNA to 10 primary murine tumors after neuraminidase pretreatment of tissue sections but greater than 50% binding in 3 of 5 metastases. These studies indicate, in both human adenocarcinomas and an experimental tumor system, that most tumor cells which metastasize show preferential binding of PNA and SBA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram J. S., Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980 Nov;11(1):63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell V. H., Crowther D., Gallagher J., Stoddart R. W. Studies of lectin binding to normal and neoplastic lymph nodes. II. Non-Hodgkin's lymphoma. Br J Cancer. 1982 Oct;46(4):582–592. doi: 10.1038/bjc.1982.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böcker W., Klaubert A., Bahnsen J., Schweikhart G., Pollow K., Mitze M., Kreienberg R., Beck T., Stegner H. E. Peanut lectin histochemistry of 120 mammary carcinomas and its relation to tumor type, grading, staging, and receptor status. Virchows Arch A Pathol Anat Histopathol. 1984;403(2):149–161. doi: 10.1007/BF00695231. [DOI] [PubMed] [Google Scholar]
- Cooper H. S. Peanut lectin-binding sites in large bowel carcinoma. Lab Invest. 1982 Oct;47(4):383–390. [PubMed] [Google Scholar]
- Cooper H. S., Reuter V. E. Peanut lectin-binding sites in polyps of the colon and rectum. Adenomas, hyperplastic polyps, and adenomas with in situ carcinoma. Lab Invest. 1983 Dec;49(6):655–661. [PubMed] [Google Scholar]
- Cummings K. B. Carcinoma of the bladder: predictors. Cancer. 1980 Apr 15;45(7 Suppl):1849–1855. [PubMed] [Google Scholar]
- Dawson P. A., Filipe M. I. An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer. 1976 May;37(5):2388–2398. doi: 10.1002/1097-0142(197605)37:5<2388::aid-cncr2820370531>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Donaghue T. P., Kerbel R. S. Membrane-associated alterations detected in poorly tumorigenic lectin-resistant variant sublines of a highly malignant and metastatic murine tumor. J Natl Cancer Inst. 1981 Jan;66(1):129–139. [PubMed] [Google Scholar]
- Fidler I. J., Hart I. R. Biological and experimental consequences of the zonal composition of solid tumors. Cancer Res. 1981 Aug;41(8):3266–3267. [PubMed] [Google Scholar]
- Fidler I. J., Nicolson G. L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976 Nov;57(5):1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- Finne J., Tao T. W., Burger M. M. Carbohydrate changes in glycoproteins of a poorly metastasizing wheat germ agglutinin-resistant melanoma clone. Cancer Res. 1980 Jul;40(7):2580–2587. [PubMed] [Google Scholar]
- Fischer J., Klein P. J., Vierbuchen M., Fischer R., Uhlenbruck G. Lectin binding properties of glycoproteins in cells of normal gastric mucosa and gastric cancers: a comparative histochemical and biochemical study. Cancer Detect Prev. 1983;6(1-2):137–147. [PubMed] [Google Scholar]
- Fogel M., Gorelik E., Segal S., Feldman M. Differences in cell surface antigens of tumor metastases and those of the local tumor. J Natl Cancer Inst. 1979 Mar;62(3):585–588. doi: 10.1093/jnci/62.3.585. [DOI] [PubMed] [Google Scholar]
- Franklin W. A. Tissue binding of lectins in disorders of the breast. Cancer. 1983 Jan 15;51(2):295–300. doi: 10.1002/1097-0142(19830115)51:2<295::aid-cncr2820510222>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Furmanski P., Kirkland W. L., Gargala T., Rich M. A. Prognostic value of concanavalin A reactivity of primary human breast cancer cells. Cancer Res. 1981 Oct;41(10):4087–4092. [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Grimes W. J. Glycosyltransferase and sialic acid levels of normal and transformed cells. Biochemistry. 1973 Feb 27;12(5):990–996. doi: 10.1021/bi00729a031. [DOI] [PubMed] [Google Scholar]
- Heppner G. H. Tumor heterogeneity. Cancer Res. 1984 Jun;44(6):2259–2265. [PubMed] [Google Scholar]
- Howard D. R., Batsakis J. G. Peanut agglutinin: a new marker for tissue histiocytes. Am J Clin Pathol. 1982 Apr;77(4):401–408. doi: 10.1093/ajcp/77.4.401. [DOI] [PubMed] [Google Scholar]
- Howard D. R., Ferguson P., Batsakis J. G. Carcinoma-associated cytostructural antigenic alterations: detection by lectin binding. Cancer. 1981 Jun 15;47(12):2872–2877. doi: 10.1002/1097-0142(19810615)47:12<2872::aid-cncr2820471220>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Ree H. J. Histochemical studies on lectin binding in reactive lymphoid tissues. J Histochem Cytochem. 1983 Apr;31(4):538–546. doi: 10.1177/31.4.6827084. [DOI] [PubMed] [Google Scholar]
- Irimura T., Nicolson G. L. Carbohydrate chain analysis by lectin binding to electrophoretically separated glycoproteins from murine B16 melanoma sublines of various metastatic properties. Cancer Res. 1984 Feb;44(2):791–798. [PubMed] [Google Scholar]
- Isaacson P., Attwood P. R. Failure to demonstrate specificity of the morphological and histochemical changes in mucosa adjacent to colonic carcinoma (transitional mucosa). J Clin Pathol. 1979 Mar;32(3):214–218. doi: 10.1136/jcp.32.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariniemi A. L., Holthöfer H., Miettinen A., Virtanen I. Altered binding of Ulex europaeus I lectin to psoriatic epidermis. Br J Dermatol. 1983 Nov;109(5):523–529. doi: 10.1111/j.1365-2133.1983.tb07674.x. [DOI] [PubMed] [Google Scholar]
- Leathem A., Dokal I., Atkins N. Lectin binding to normal and malignant breast tissue. Diagn Histopathol. 1983 Jul-Dec;6(3-4):171–180. [PubMed] [Google Scholar]
- Lehman T. P., Cooper H. S., Mulholland S. G. Peanut lectin binding sites in transitional cell carcinoma of the urinary bladder. Cancer. 1984 Jan 15;53(2):272–277. doi: 10.1002/1097-0142(19840115)53:2<272::aid-cncr2820530215>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Foley J., Judd W. J. Peanut lectin agglutinin and alpha-lactalbumin. Binding and immunohistochemical localization in breast tissues. Arch Pathol Lab Med. 1984 May;108(5):392–395. [PubMed] [Google Scholar]
- Louis C. J., Sztynda T., Cheng Z. M., Wyllie R. G. Lectin-binding affinities of human breast tumors. Cancer. 1983 Oct 1;52(7):1244–1250. doi: 10.1002/1097-0142(19831001)52:7<1244::aid-cncr2820520719>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Louis C. J., Wyllie R. G., Chou S. T., Sztynda T. Lectin-binding affinities of human epidermal tumors and related conditions. Am J Clin Pathol. 1981 May;75(5):642–647. doi: 10.1093/ajcp/75.5.642. [DOI] [PubMed] [Google Scholar]
- Mitelman F. The chromosomes of fifty primary Rous rat sarcomas. Hereditas. 1971;69(2):155–186. doi: 10.1111/j.1601-5223.1971.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Nemanic M. K., Whitehead J. S., Elias P. M. Alterations in membrane sugars during epidermal differentiation: visualization with lectins and role of glycosidases. J Histochem Cytochem. 1983 Jul;31(7):887–897. doi: 10.1177/31.7.6854004. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Klein P. J., Rudland P. S. Binding of peanut lectin to breast epithelium, human carcinomas, and a cultured rat mammary stem cell: use of the lectin as a marker of mammary differentiation. J Natl Cancer Inst. 1979 Dec;63(6):1339–1346. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Sci Am. 1979 Mar;240(3):66–76. doi: 10.1038/scientificamerican0379-66. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Lotan R., Ravid A., Sharon N. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J Immunol. 1975 Nov;115(5):1243–1248. [PubMed] [Google Scholar]
- Orgad U., Alroy J., Ucci A., Merk F. B. Histochemical studies of epithelial cell glycoconjugates in atrophic, metaplastic, hyperplastic, and neoplastic canine prostate. Lab Invest. 1984 Mar;50(3):294–302. [PubMed] [Google Scholar]
- Reano A., Faure M., Jacques Y., Reichert U., Schaefer H., Thivolet J. Lectins as markers of human epidermal cell differentiation. Differentiation. 1982;22(3):205–210. doi: 10.1111/j.1432-0436.1982.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Ree H. J. Lectin histochemistry of malignant tumors. II. Concanavalin A: a new histochemical marker for macrophage-histiocytes in follicular lymphoma. Cancer. 1983 May 1;51(9):1639–1646. doi: 10.1002/1097-0142(19830501)51:9<1639::aid-cncr2820510915>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ree H. J., Raine L., Crowley J. P. Lectin binding patterns in diffuse large cell lymphoma. Cancer. 1983 Dec 1;52(11):2089–2099. doi: 10.1002/1097-0142(19831201)52:11<2089::aid-cncr2820521121>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Rittman B. R., Mackenzie I. C. Effects of histological processing on lectin binding patterns in oral mucosa and skin. Histochem J. 1983 May;15(5):467–474. doi: 10.1007/BF01002700. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Malchiodi F. Binding of peanut lectin to thymic cortex and germinal centres of lymphoid tissue. Immunology. 1981 Apr;42(4):583–591. [PMC free article] [PubMed] [Google Scholar]
- Shapiro J., Jersky J., Katzav S., Feldman M., Segal S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981 Sep;68(3):678–685. doi: 10.1172/JCI110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Lectins. Sci Am. 1977 Jun;236(6):108-16, 118-9. doi: 10.1038/scientificamerican0677-108. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Nicolson G. L. Cell surface glycoproteins of 13762NF mammary adenocarcinoma clones of differing metastatic potentials. Exp Cell Res. 1983 Sep;147(2):255–267. doi: 10.1016/0014-4827(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Tao T. W., Jenkins J. M., Vosbeck K., Matter A., Miller M., Jockusch B. M., Shen Z. H., Burger M. M. Lectin-resistant variants of mouse melanoma cells. II. In vitro characteristics. Int J Cancer. 1983 Feb 15;31(2):239–247. doi: 10.1002/ijc.2910310218. [DOI] [PubMed] [Google Scholar]
- Teshima S., Hirohashi S., Shimosato Y., Kishi K., Ino Y., Matsumoto K., Yamada T. Histochemically demonstrable changes in cell surface carbohydrates of human germ cell tumors. Lab Invest. 1984 Mar;50(3):271–277. [PubMed] [Google Scholar]
- Vierbuchen M., Klein P. J., Rösel S., Fischer J. Peanut agglutinin (PNA) binding sites: a useful marker for hormonal dependence in experimental breast cancer. Cancer Detect Prev. 1983;6(1-2):207–214. [PubMed] [Google Scholar]
- Volk T., Geiger B., Raz A. Motility and adhesive properties of high- and low-metastatic murine neoplastic cells. Cancer Res. 1984 Feb;44(2):811–824. [PubMed] [Google Scholar]
- Walker R. A. The binding of peroxidase-labelled lectins to human breast epithelium. I--Normal, hyperplastic and lactating breast. J Pathol. 1984 Apr;142(4):279–291. doi: 10.1002/path.1711420406. [DOI] [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]
- Yonezawa S., Nakamura T., Tanaka S., Sato E. Glycoconjugate with Ulex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst. 1982 Oct;69(4):777–785. [PubMed] [Google Scholar]