Abstract
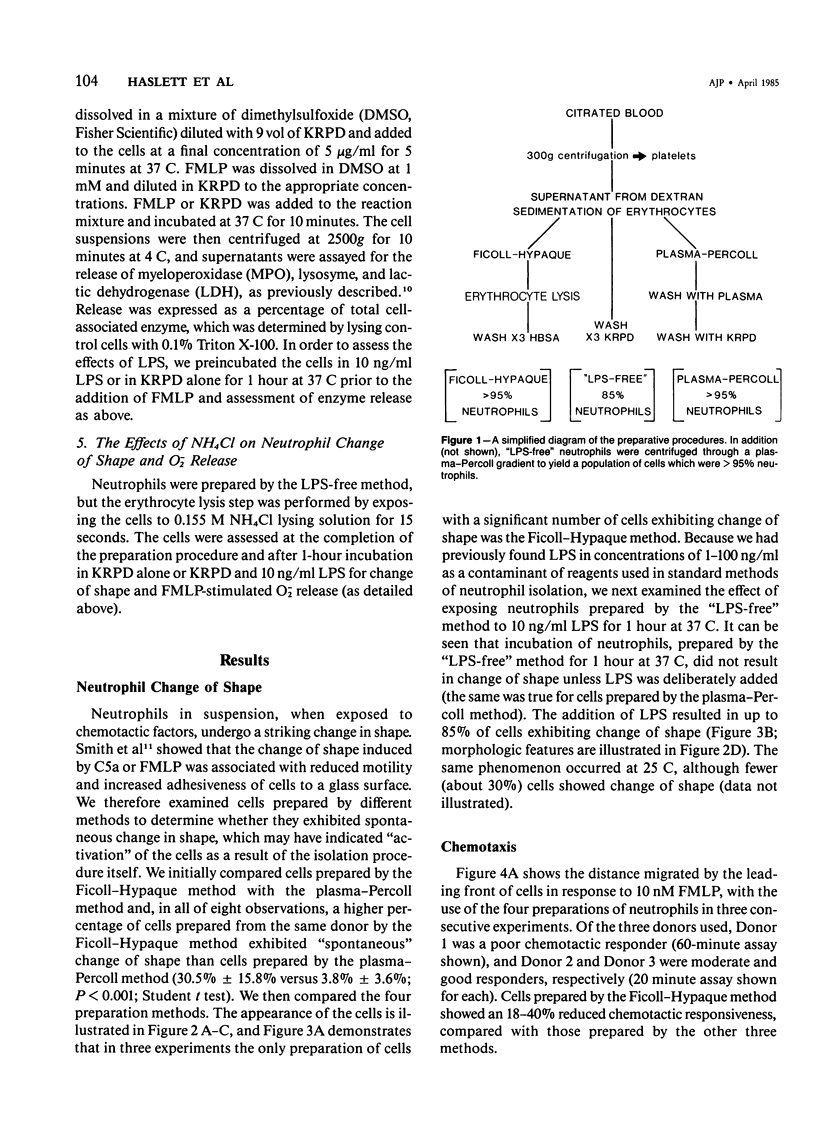
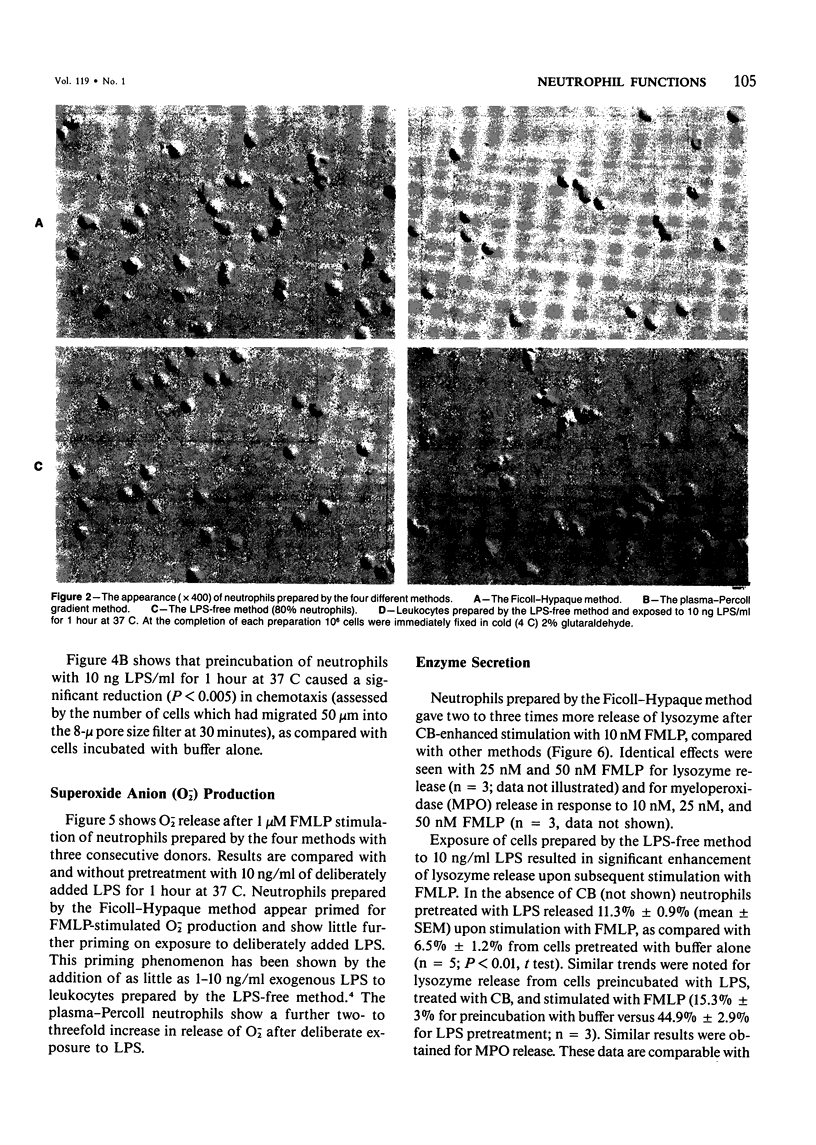
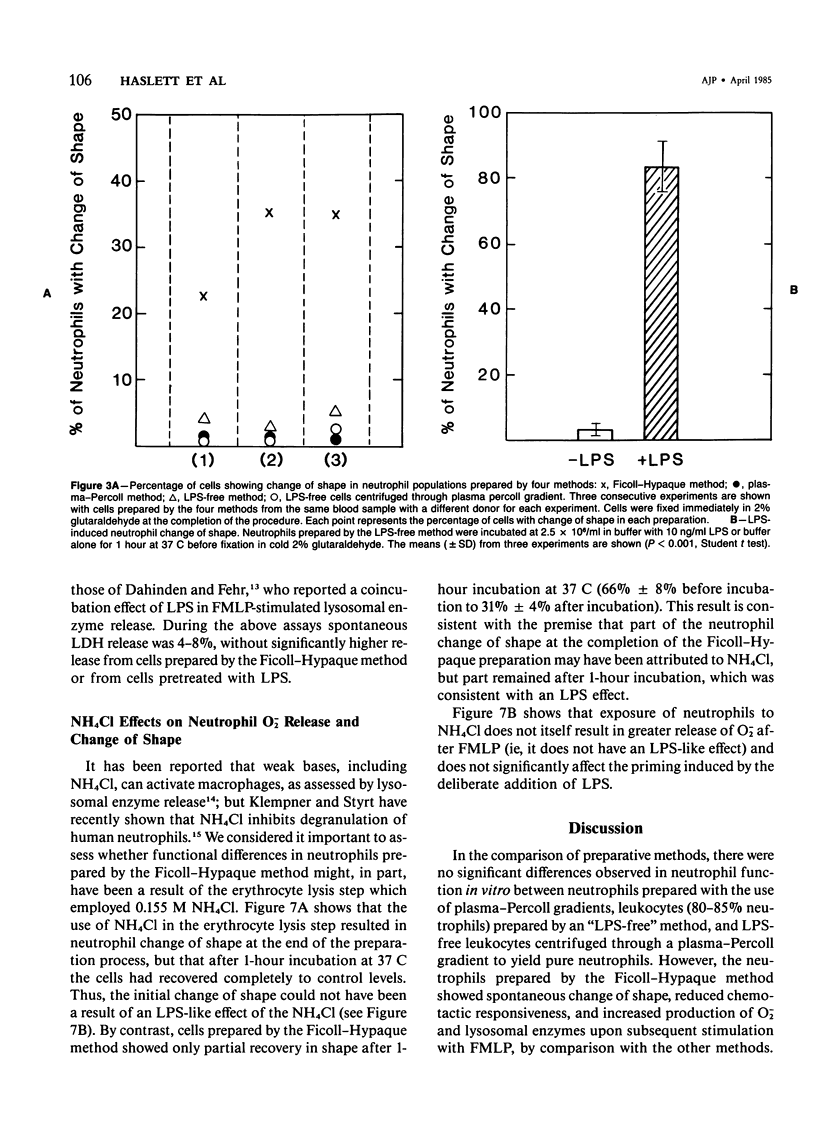
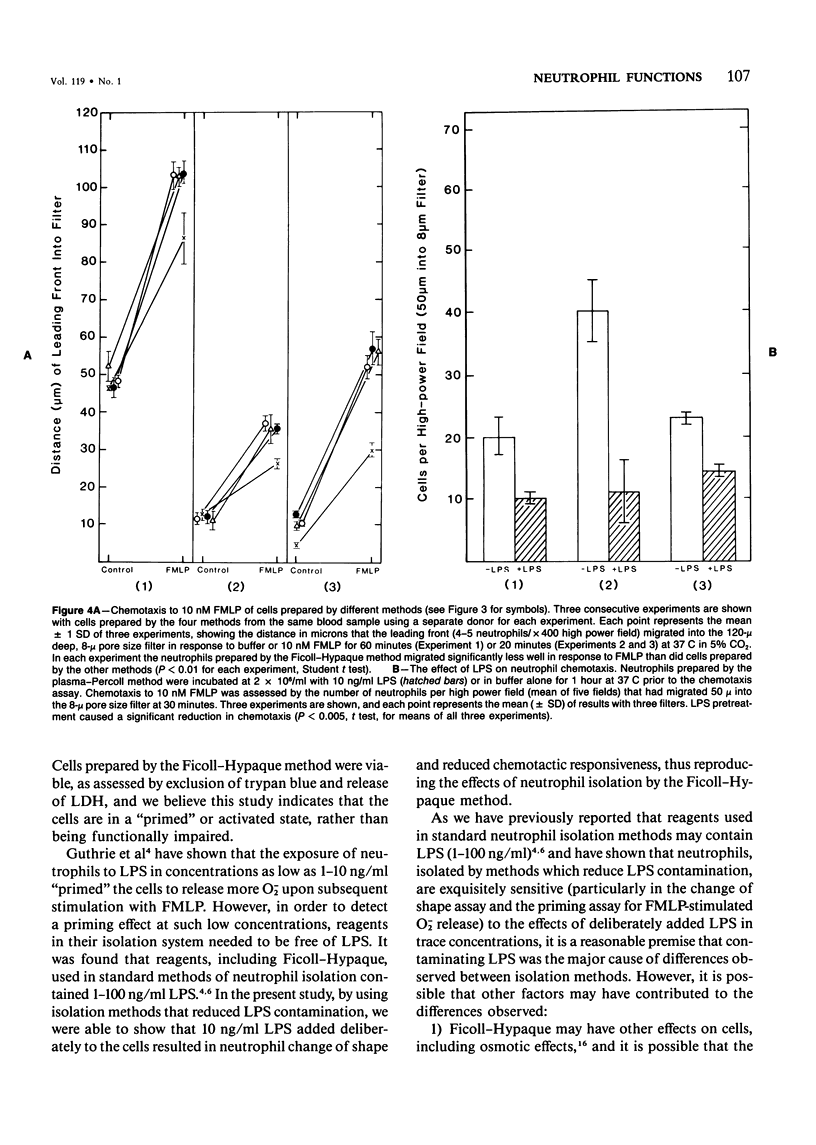
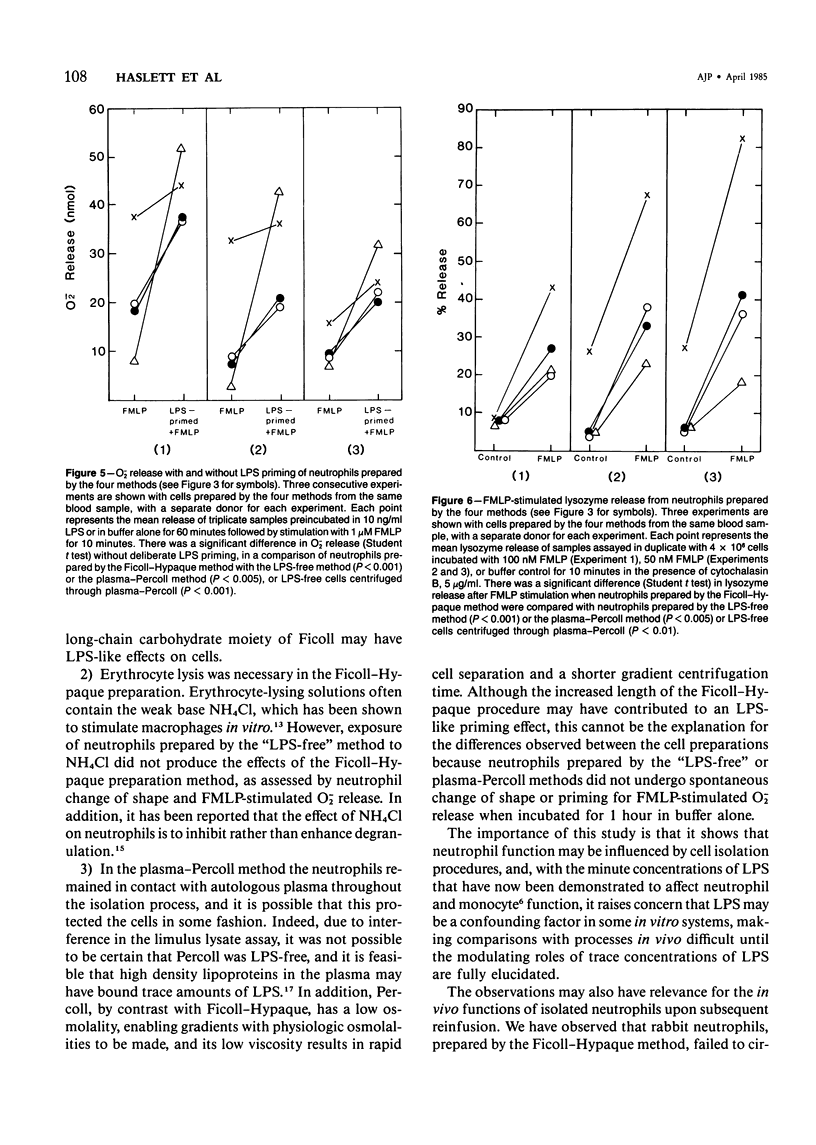
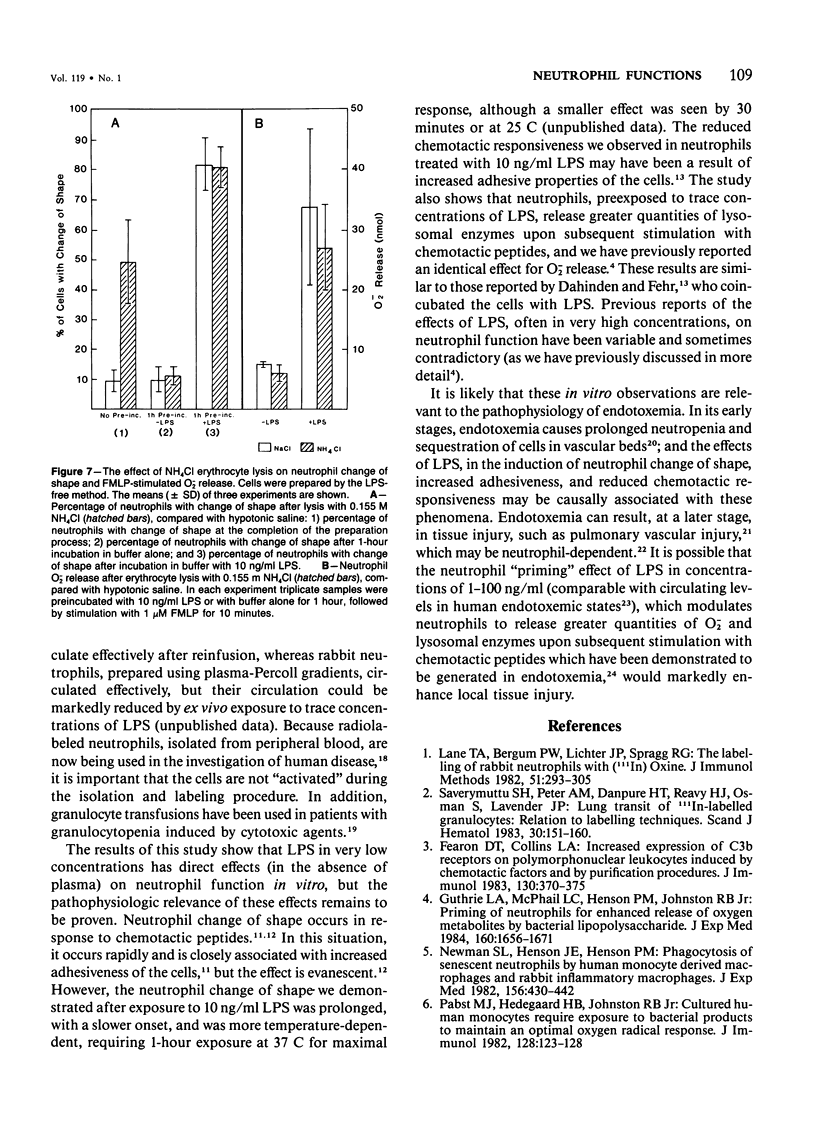
Human neutrophils were isolated from peripheral blood by four methods: 1) Ficoll-Hypaque gradients and erythrocyte lysis, 2) plasma-Percoll gradients, 3) a "lipopolysaccharide (LPS)-free" method yielding 85% neutrophils, and 4) by centrifugation of cells prepared by Method 3 through a plasma-Percoll gradient to produce pure neutrophils. The use of the Ficoll-Hypaque method resulted in spontaneous change of cell shape, enhanced formyl-methionyl-leucyl-phenylalanine (FMLP)-stimulated release of superoxide anion, increased release of lysosomal enzymes upon subsequent FMLP stimulation, and reduced chemotactic responsiveness, by comparison with the other methods. These effects were not due to erythrocyte lysis by NH4C1 but were reproduced by exposure of neutrophils prepared by the "LPS-free" method or the use of plasma-Percoll gradients to 10-100 ng/ml LPS. Neutrophil change of shape and stimulated O-2 production were particularly sensitive markers of these effects. The effects of trace concentrations of LPS in the modulation of neutrophil function may have relevance to the pathophysiology of endotoxemia and its resultant tissue injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigham K. L., Bowers R., Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979 Aug;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- Danpure H. J., Osman S., Brady F. The labelling of blood cells in plasma with 111In-tropolonate. Br J Radiol. 1982 Mar;55(651):247–249. doi: 10.1259/0007-1285-55-651-247. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hoffstein S. T., Friedman R. S., Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982 Oct;95(1):234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Gerber H., Hess M. W., Cottier H. Studies on the regulation of the neutrophil chemotactic response using a rapid and reliable method for measuring random migration and chemotaxis of neutrophil granulocytes. Agents Actions. 1976 Feb;6(1-3):326–339. doi: 10.1007/BF01972250. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. J Clin Invest. 1983 Nov;72(5):1793–1800. doi: 10.1172/JCI111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. A., Bergum P. W., Lichter J. P., Spragg R. G. The labeling of rabbit neutrophils with [111In]oxine. J Immunol Methods. 1982;51(3):293–305. doi: 10.1016/0022-1759(82)90396-9. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Primary amines induce selective release of lysosomal enzymes from mouse macrophages. Biochem J. 1980 Jun 15;188(3):933–936. doi: 10.1042/bj1880933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Danpure H. J., Reavy H. J., Osman S., Lavender J. P. Lung transit of 111Indium-labelled granulocytes. Relationship to labelling techniques. Scand J Haematol. 1983 Feb;30(2):151–160. doi: 10.1111/j.1600-0609.1983.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Hodgson H. J., Chadwick V. S., Lavender J. P. Indium-111 autologous leucocyte scanning: comparison with radiology for imaging the colon in inflammatory bowel disease. Br Med J (Clin Res Ed) 1982 Jul 24;285(6337):255–257. doi: 10.1136/bmj.285.6337.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. G., Connett J. E., Gale R. P., Bloomfield C. D., Herzig G. P., McCullough J., Maguire L. C., Winston D. J., Ho W., Stump D. C. A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. N Engl J Med. 1981 Sep 10;305(11):597–603. doi: 10.1056/NEJM198109103051101. [DOI] [PubMed] [Google Scholar]
- Wells J. R., Opelz G., Cline M. J. Characterization of functionally distinct lymphoid and myeloid cells from human blood and bone marrow. I. Separation by a buoyant density gradient technique. J Immunol Methods. 1977;18(1-2):63–77. doi: 10.1016/0022-1759(77)90159-4. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



