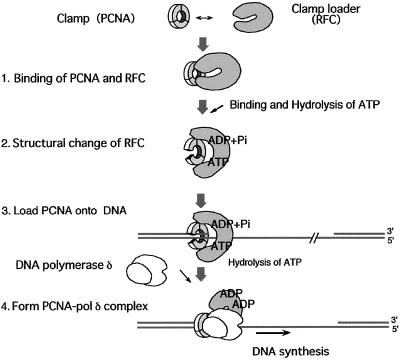
Figure 6.
A model for PCNA loading driven by RFC structural change. RFC associates with PCNA independent of ATP and changes its structure on binding and hydrolysis of ATP. Because both the prominent change in the structure of RFC and the loading of a functional PCNA clamp occur concomitantly by hydrolysis of ATP, the PCNA ring may open during the structural change. Some change in the PCNA structure may occur by binding of ATP, but the intermediate state formed during ATP hydrolysis produces the most pronounced structural change, to the C form, in which two fingers of RFC can hold PCNA strongly. This conformational change is proposed to open the PCNA ring to form a functional PCNA clamp on DNA. The C-form state of RFC corresponds either to an intermediate with bound ADP + Pi or to one with ATP hydrolysis of only some of the multiple bound ATPs. If the latter is the case, individual subunits might change their structure by stepwise hydrolysis of ATP, and the accumulation of this change would gradually open the whole RFC molecule to the C form. Through the structural change, the affinity between RFC and PCNA is decreased, and a functional PCNA clamp is formed on DNA, to which DNA polymerase δ is recruited. The number of ATPs that appear in this figure is arbitrary.