Abstract
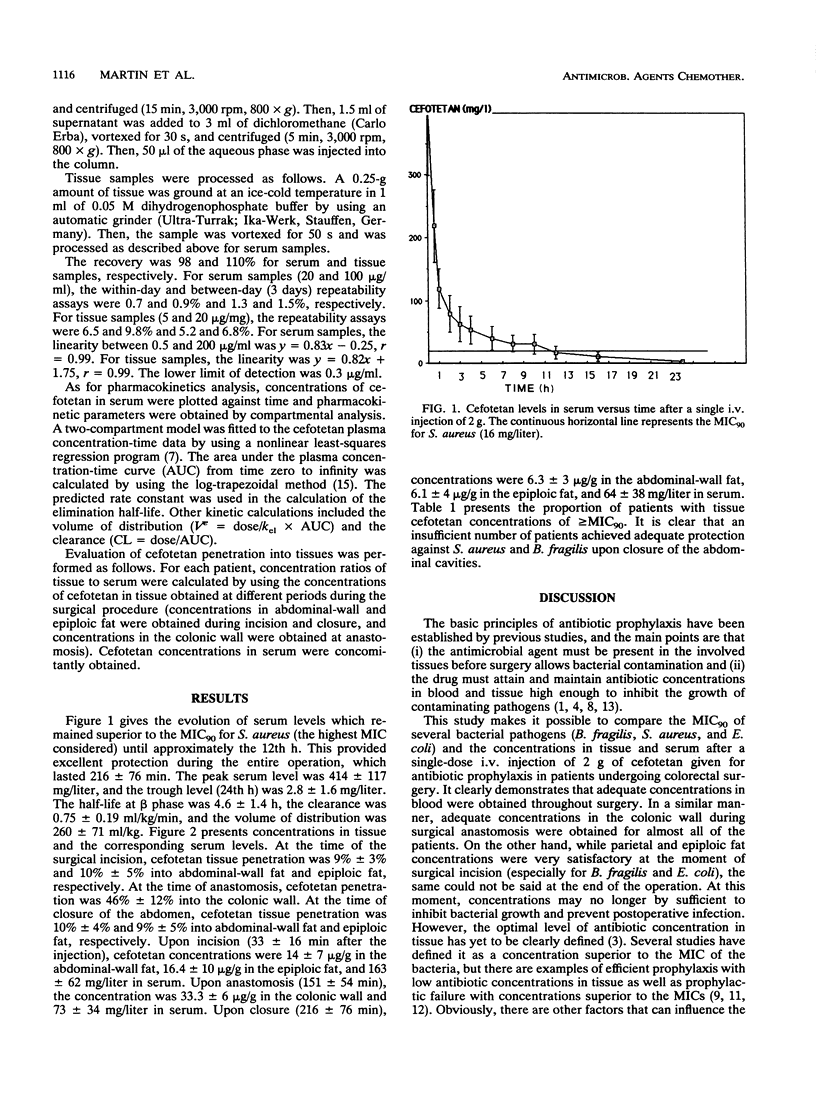
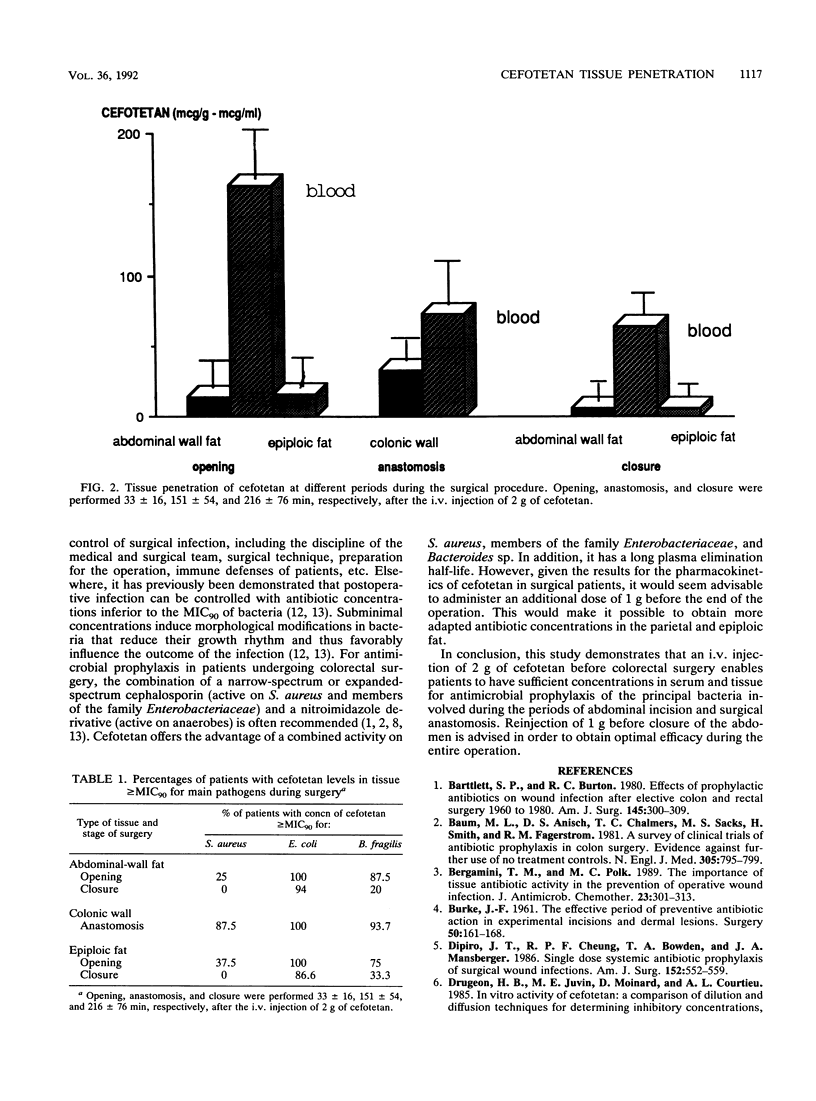
The pharmacokinetics and tissue penetration of cefotetan were studied after a single injection of 2 g given intravenously for antimicrobial prophylaxis to 16 consecutive patients undergoing colorectal surgery. Concentrations in tissue greater than or equal to the MIC for 90% of the main pathogens tested were considered adequate. The elimination half-life at beta phase was 4.6 +/- 1.4 h, the total body clearance was 0.75 +/- 0.19 ml/kg/min, and the volume of distribution was 260 +/- 71 ml/kg. At the time of incision (33 +/- 16 min after the injection), cefotetan concentrations were 14.2 +/- 7 micrograms/g in abdominal-wall fat, 16.4 +/- 1 micrograms/g in epiploic fat, and 163 +/- 62 mg/liter in serum. At the time of surgical anastomosis (151 +/- 54 min), cefotetan concentrations were 33.3 +/- 6 micrograms/g in the colonic wall and 73 +/- 34 mg/liter in serum. Upon closure of the abdomen (216 +/- 76 min), cefotetan concentrations were 6.3 +/- 3 micrograms/g in abdominal-wall fat, 6.1 +/- 4 micrograms/g in epiploic fat, and 64 +/- 38 mg/liter in serum. Cefotetan tissue penetration was 10% into abdominal and epiploic fat and 46% into the colonic wall. Levels in tissue were compared with the MIC for 90% of the most frequently encountered pathogenic germs (Staphylococcus aureus, Bacteroides fragilis, and Escherichia coli). Adequate concentrations in tissue were obtained up to anastomosis but not upon closure. The authors therefore recommend the injection of an additional dose of 1 g before closure in order to ensure optimal efficacy throughout the surgical procedure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett S. P., Burton R. C. Effects of prophylactic antibiotics on wound infection after elective colon and rectal surgery: 1960 to 1980. Am J Surg. 1983 Feb;145(2):300–309. doi: 10.1016/0002-9610(83)90088-0. [DOI] [PubMed] [Google Scholar]
- Baum M. L., Anish D. S., Chalmers T. C., Sacks H. S., Smith H., Jr, Fagerstrom R. M. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med. 1981 Oct 1;305(14):795–799. doi: 10.1056/NEJM198110013051404. [DOI] [PubMed] [Google Scholar]
- Bergamini T. M., Polk H. C., Jr The importance of tissue antibiotic activity in the prevention of operative wound infection. J Antimicrob Chemother. 1989 Mar;23(3):301–313. doi: 10.1093/jac/23.3.301. [DOI] [PubMed] [Google Scholar]
- Burke J. F. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961 Jul;50:161–168. [PubMed] [Google Scholar]
- DiPiro J. T., Cheung R. P., Bowden T. A., Jr, Mansberger J. A. Single dose systemic antibiotic prophylaxis of surgical wound infections. Am J Surg. 1986 Nov;152(5):552–559. doi: 10.1016/0002-9610(86)90228-x. [DOI] [PubMed] [Google Scholar]
- Kaiser A. B. Antimicrobial prophylaxis in surgery. N Engl J Med. 1986 Oct 30;315(18):1129–1138. doi: 10.1056/NEJM198610303151805. [DOI] [PubMed] [Google Scholar]
- Kosmidis J., Stathakis C., Mantopoulos K., Pouriezi T., Papathanassiou B., Daikos G. K. Clinical pharmacology of cefotaxime including penetration into bile, sputum, bone and cerebrospinal fluid. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):147–151. doi: 10.1093/jac/6.suppl_a.147. [DOI] [PubMed] [Google Scholar]
- Lorian V. Low concentrations of antibiotics. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):15–26. doi: 10.1093/jac/15.suppl_a.15. [DOI] [PubMed] [Google Scholar]
- Mutch D., Richards G., Brown R. A., Mulder D. S. Bioactive antibiotic levels in the human aorta. Surgery. 1982 Dec;92(6):1068–1071. [PubMed] [Google Scholar]
- Pitt H. A., Roberts R. B., Johnson W. D., Jr Gentamicin levels in the human biliary tract. J Infect Dis. 1973 Mar;127(3):299–302. doi: 10.1093/infdis/127.3.299. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jr, Jacobus N. V., Gorbach S. L., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P. Nationwide study of the susceptibility of the Bacteroides fragilis group in the United States. Antimicrob Agents Chemother. 1985 Nov;28(5):675–677. doi: 10.1128/aac.28.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd The effects and significance of subminimal inhibitory concentrations of antibiotics. Rev Infect Dis. 1979 Sep-Oct;1(5):781–786. doi: 10.1093/clinids/1.5.781. [DOI] [PubMed] [Google Scholar]
- Zak O., Kradolfer F. Effects of subminimal inhibitory concentrations of antibiotics in experimental infections. Rev Infect Dis. 1979 Sep-Oct;1(5):862–879. doi: 10.1093/clinids/1.5.862. [DOI] [PubMed] [Google Scholar]


