Figure 1.
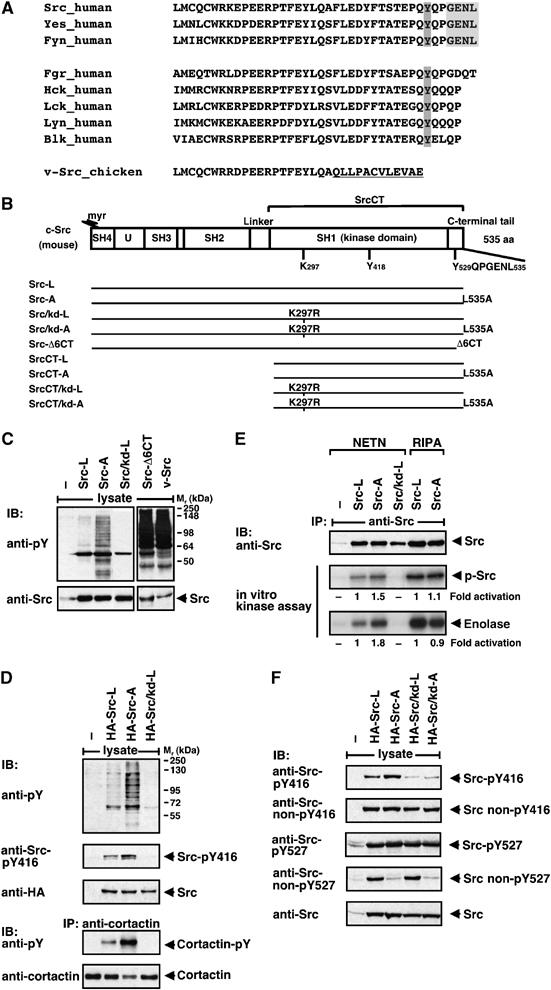
Regulation of c-Src by its very C-terminal sequence. (A) C-terminal sequence alignment of Src-family kinases. The PDZ binding site is depicted in light grey and the regulatory Tyr527 in dark grey. The v-Src-specific C-terminus is underlined. (B) Domain structure of murine c-Src. The molecule is composed of an N-terminal Src homology (SH)4 domain attached with a myristoyl group (myr), a unique region (U), SH3 and SH2 protein–protein interaction domains, a kinase domain (SH1) that contains Lys297 and Tyr418 (Tyr416 in chicken) and a C-terminal regulatory tail that contains Tyr529 (Tyr527 in chicken) and a PDZ binding sequence (GENL). The regions encoded by the different Src constructs are shown. (C–F) Regulation of Src by its C-terminal sequence. HEK 293 cells were transiently transfected with empty vector (−) or constructs, as indicated. Lysates of starved cells were analysed by blotting (IB) with anti-phospho-tyrosine (pY), anti-Src-pY416, anti-Src non-pY416, anti-Src-pY527 and anti-Src non-pY527 antibodies. Src expression was controlled by blotting with anti-Src or anti-HA antibody. (D) For analysis of cortactin phosphorylation, lysates were precipitated (IP) with anti-cortactin antibody and blotted with anti-pY and anti-cortactin antibodies. (E) Immunopurified Src proteins were analysed by in vitro kinase assay for autophosphorylation (p-Src) and for substrate phosphorylation (enolase). Numbers indicate fold activation normalized to Src expression levels.
