Figure 3.
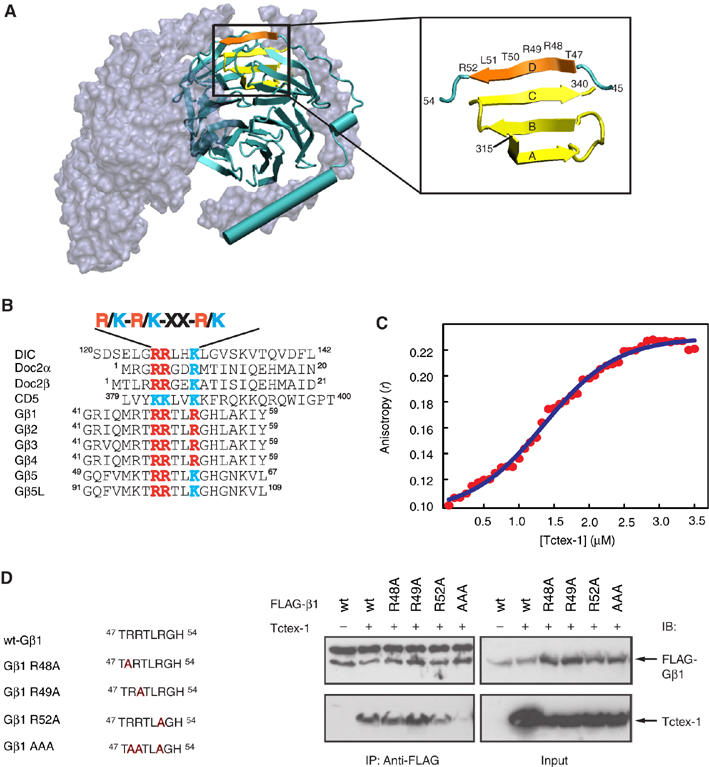
Analysis of the Tctex-1-interacting motif on Gβ. (A) Structure of heterotrimer Gαβγ highlighting the Tctex-1-binding motif in Gβ. Gα and Gγ (gray) are shown as molecular surface representation, whereas Gβ (cyan) is shown as a secondary structure cartoon. Four β-strands that make up blade 7 of the Gβ propeller are highlighted (β-strands A–C in yellow and β-strand D in orange, left panel). Expanded region (right panel) shows the region of Gβ involved in the formation of blade 7. C-terminal residues 315–340 (yellow) form the A, B and C β-strands, whereas N-terminal residues 47–52 (orange) form the outermost D β-strand of this blade. Residues 47–52 comprise the consensus R/K-R/K-X-X-R/K Tctex-1-binding motif. Image created using VMD software (Humphrey et al, 1996). Crystal coordinates obtained from PDB file 1GOT (Lambright et al, 1996). (B) Consensus sequence R/K-R/K-X-X-R/K found in various Tctex-1-interacting proteins. (C) Fluorescence anisotropy of MIANS-labeled Gβ-peptide in the presence of Tctex-1. Tctex-1 was titrated to the MIANS Gβ-peptide, whose concentration was maintained at 0.7 μM. With increasing concentration of Tctex-1, there was an enhancement in the anisotropy value, indicative of specific binding of the peptide to the protein. In addition, the plot showed a sigmoidal curve, indicative of cooperative binding. Each data point is an average of five readings with standard error less than 5%. The Kd value for the Tctex-1-Gβ–peptide interaction from nonlinear regression analysis was calculated to be 1.35 μM. (D) Reduced interaction of Gβ AAA mutant with Tctex-1. HEK cells were cotransfected with Tctex-1 and the indicated FLAG-tagged Gβ mutants. The AAA Gβ1 mutant, wherein all three Arg residues (R48, R49 and R52) within the consensus Tctex-1-binding motif are mutated to alanine, showed significantly reduced ability to co-IP Tctex-1. The upper band observed in the top left panel of the anti-FLAG IP corresponds to the IgG heavy chain. This image is representative of three independent experiments.
