Figure 2.
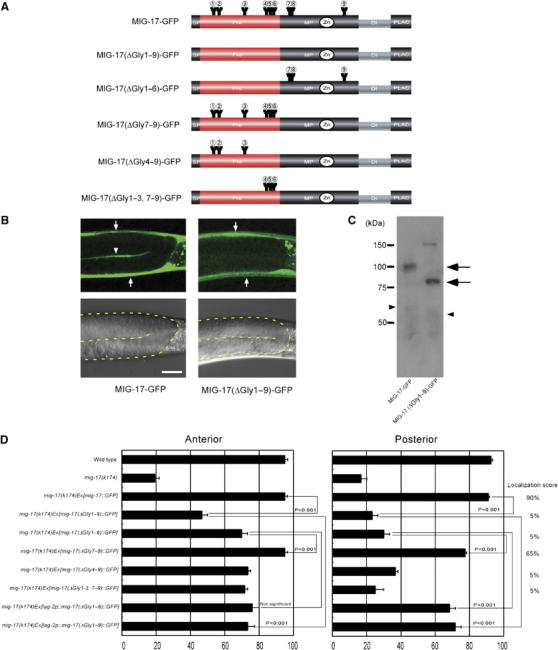
Requirement of N-glycosylation for MIG-17 localization and function. (A) Glycosylation mutant constructs. Only the intact potential glycosylation sites are indicated. All asparagines of potential N-glycosylation sites were changed to glutamines in MIG-17(ΔGly1–9)-GFP: N52Q (AAT to CAA), N65Q (AAC to CAA), N123Q (AAT to CAA), N172Q (AAT to CAA), N183Q (AAC to CAA), N189Q (AAT to CAA), N218Q (AAC to CAA), N219Q (AAT to CAA) and N350Q (AAT to CAA). (B) Confocal (upper) and Nomarski (lower) images of wild-type hermaphrodites expressing MIG-17-GFP (left) or MIG-17(ΔGly1–9)-GFP (right). The boundaries of the gonads are depicted by a dotted line in the Nomarski images. Lateral views of posterior gonads. Dorsal to the top, anterior to the left. The gonadal localization of MIG-17 can be detected by linear GFP fluorescence between the proximal and distal gonad arms in animals expressing MIG-17-GFP (arrowhead). The strong dorsal and ventral GFP fluorescence corresponds to expression of transgenes in the body wall muscles (arrows). Punctate fluorescence outside the gonads is gut autofluorescence. Scale bar, 20 μm. (C) Western blot analysis. The lysates from wild-type worms expressing MIG-17-GFP or MIG-17(ΔGly1–9)-GFP were immunoblotted with anti-GFP. Pro- and mature forms are shown by arrows and arrowheads, respectively. The band of about 150 kDa in the MIG-17(ΔGly1–9)-GFP lane is often detected even in non-transgenic worms and probably due to nonspecific binding of the secondary antibody. (D) Requirement for glycosylation of MIG-17 in MIG-17 localization and function. Data for DTC migration are shown as in Figure 1D (n=120). Percentage of posterior gonads localized with GFP is indicated as the localization score on the right (n=20).
