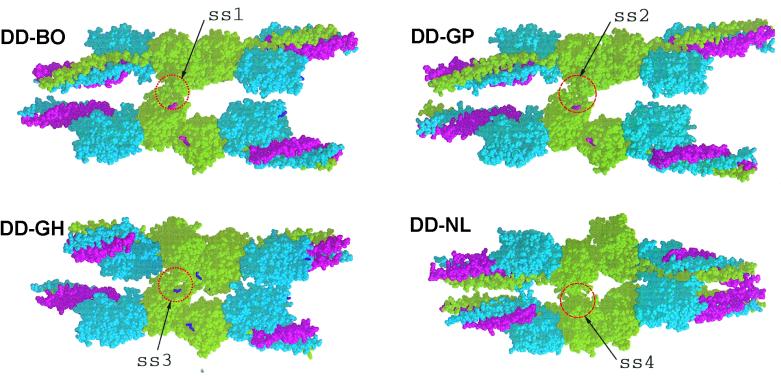
Figure 1.
Four DD structures: DD-BO (PDB code 1FZC); DD-GP (PDB code 1FZB); DD-GH (PDB code 1FZF); and DD-NL (PDB code 1FZE) showing crystal packing with neighboring molecules. The lateral associations (ss = side-by-side) involving γC domains are circled and denoted ss1, ss2, ss3, and ss4. The two structures with GPR- knobs in the γ-chain holes (DD-BO and DD-GP) are virtually identical. When the same holes are occupied by Gly-His-Arg knobs (DD-GH), the lateral interaction is slightly shifted. When these holes are unoccupied (DD-NL), the packing is completely different (ss4), the adjacent domains being greatly rotated relative to each other compared with all of the other structures. Red, α-chains; blue, β-chains; green, γ-chains. The peptide ligand GPRPam (A knob) is shown in magenta; the B knob (GHRPam) is shown in purple.