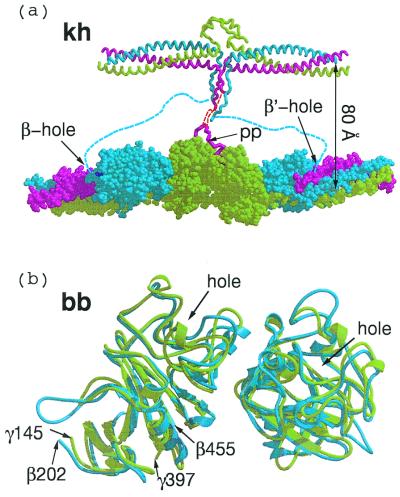
Figure 3.
(a) Knob–hole interactions (kh) between central domain (Top) and γC and βC domains of associated molecules within a protofibril. The 80 Å-distance between the two strands of the protofibril is an approximation based on extension of α-chain residues 34 to 17, which were not clearly delineated in the chicken fibrinogen structure (red dashed line). Flexible amino-terminal regions of β chains (blue dashed line) may allow B knobs to reach β-chain holes of the same companion molecule in protofibril clasper-style. (b) Superposition of two computer-associated βC domains (blue) on homologous γC domains (light green) as they occur in crosslinked fibrin (DD-BO, PDB-code 1FZC).