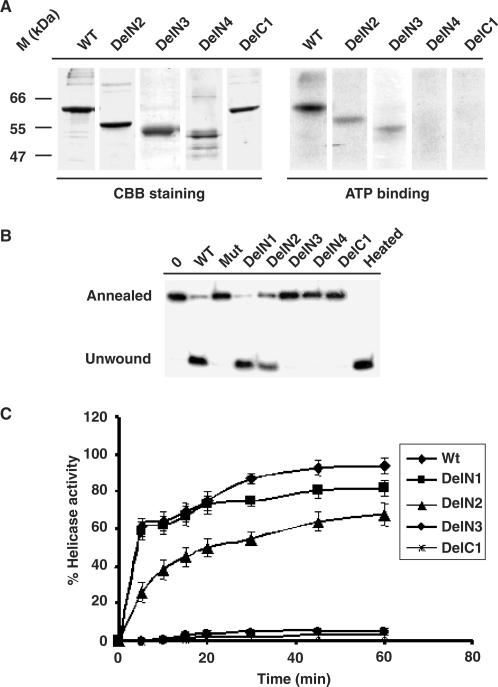
Figure 3.
ATP-binding and helicase activity of HpDnaBWt and mutant proteins. (A) ATP binding activity of various HpDnaB forms. Total 1 μg of each protein as indicated on the top was incubated in the presence of radio-labeled α-32P ATP followed by SDS-PAGE analysis of ATP bound protein by autoradiography. The right panel shows that only wild-type, DelN2 and DelN3 bind to ATP whereas DelN4 and DelC1 fail to bind ATP. The left panel shows the coomassie-stained image of the same gel. (B) Helicase activity of various HpDnaB forms. Approximately 500 nM of each protein as indicated on the top was incubated in a reaction mixture containing end-labeled 29 nucleotides probe hybridized to M13 single-stranded DNA for 30 min and the release of the unannealed probe was monitored following PAGE analysis. Annealed and unwound substrates are marked. The sample marked as ‘heated’ was boiled for 3 min before loading. Deletion mutants DelN1-N2 show the release of unwound oligo whereas DelN3-4, DelC1 and HpDnaBMut do not show any activity. Control lane does not contain any protein. (C) Time-course of helicase activity. The helicase activity of each protein as indicated in the figure was monitored every 5 min interval up to 1 h and the intensity of released unwound oligo in each case was quantified using densitometry scanning and subsequently plotted. Helicase reactions were repeated at least three times for each time point.