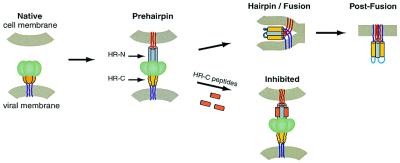
Figure 2.
A model for virus–cell membrane fusion (24). The model shown is based primarily on studies of the HIV-1 gp41-mediated membrane fusion process. The HRSV F protein likely undergoes similar conformational changes. In the native state, the fusion peptide (not shown) is inaccessible. Upon activation, the fusion protein undergoes a conformational change to the prehairpin intermediate, in which the fusion peptide (red lines) is inserted into the target-cell membrane, and the HR-N peptide region (gray) is a trimeric coiled coil. The HR-C peptide region (yellow) has not yet associated with the N peptide coiled coil. This intermediate is vulnerable to HR-C peptide inhibition (Lower, inhibitory peptides shown in orange). The prehairpin intermediate resolves to the fusion-active hairpin structure when the HR-C peptide region binds to the HR-N peptide coiled coil and adopts a helical conformation. This rearrangement results in membrane apposition. Whether hairpin formation precedes the actual membrane-fusion event or occurs simultaneously with fusion is unknown. Figure was adapted from Chan and Kim (24).