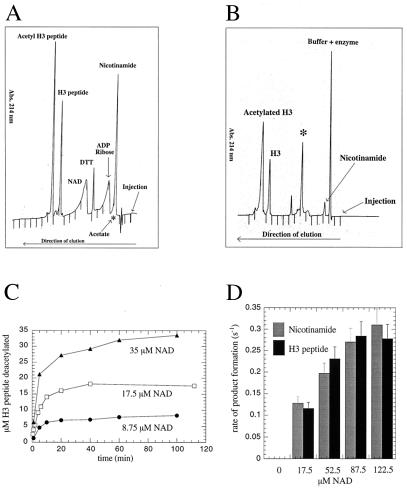
Figure 1.
HPLC analysis of the HST2 NAD-dependent deacetylation reaction. (A) Reverse-phase HPLC elution of substrate standards (NAD+ and AcLys-14 H3 peptide) and of potential products (H3 peptide, acetate, ADP-ribose, and nicotinamide). Approximately 1.5 nmol AcLys-14 H3 peptide, 1.0 nmol H3 peptide, 10 nmol NAD, 15 nmol ADP-ribose, and 15 nmol nicotinamide were mixed and subjected to reverse-phase chromatography. In a separate HPLC run, 1.0 nmol sodium [3H]acetate (100,000 cpm/nmol) was subjected to chromatography. Order of elution: nicotinamide, acetate, ADP-ribose, NAD+, H3 peptide, and AcLys-14 H3 peptide. The elution position of acetate (∗) was determined by using authentic [3H]acetate and detection by liquid scintillation counting. All others were detected by UV absorption at 214 nm. (B) Elution of products from the HST2 NAD-dependent deacetylation reaction, detected by UV absorbance at 214 nm. ∗, Previously unidentified product. Conditions: 3.6 μM HST2/175 μM NAD+/525 μM Lys-14 AcH3/1 mM DTT for 30 min at 37°C before quenching with TFA to final concentration of 1%. (C) Amount of deacetylation correlates exactly with the consumption of NAD+. This graph displays the progress curves of deacetylation at fixed but limiting [NAD+]. HST2 reaction was allowed to proceed to completion under various limiting [NAD+], and the amount of deacetylated H3 peptide was determined. Conditions: 375 nM HST2/175 μM Lys-14 AcH3/8.75, 17.5, or 35 μM NAD+/1 mM DTT for 20 min at 37°C before quenching with TFA to 1%. (D) Steady-state rate of nicotinamide and deacetylated H3 peptide formation at various fixed [NAD+]. Conditions: 375 nM HST2/175 μM Lys-14 AcH3/8.75 μM–280 μM NAD+/1 mM DTT for 1 min at 37°C before quenching with TFA to a final concentration of 1%.