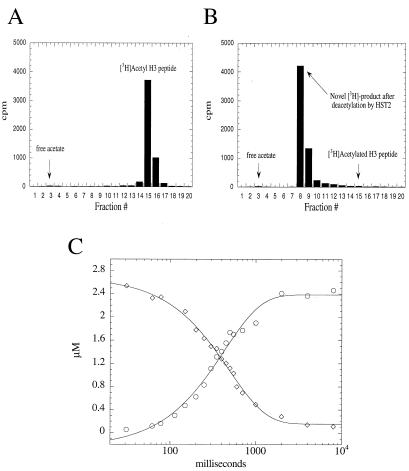
Figure 2.
Acetate is not a primary product of HST2-catalyzed histone/protein deacetylation. H3 peptide was stoichiometrically acetylated on Lys-14 by the histone acetyltransferase P/CAF and [3H]AcCoA. Monoacetylated [3H]AcLys-14 H3 peptide was then purified by HPLC (A) and used as a substrate in the HST2 deacetylation reactions (B). On complete consumption of [3H]AcLys-14 H3 by HST2, all of the original 3H from H3 peptide was converted to a labeled product that eluted much later than authentic acetate. Conditions: 375 nM HST2/175 μM NAD+/5 μM [3H]Lys-14 AcH3/1 mM DTT for 1 min at 37°C before quenching with TFA to a final concentration of 1%. (C) Single turnover rapid quench-flow analysis. HST2 (10 μM) and NAD+ (300 μM) were mixed rapidly with 2.5 μM [3H]AcLys-14 H3 peptide at 22 ± 3°C (pH 7.5) in a Hi-Tech rapid quench-flow device, RQF-63. Between 31 ms and 8 s, reactions were quenched with TFA (1%). Quantification of [3H]AcLys-14 H3 peptide (⋄) and the [3H]acetate adduct (○) was accomplished by liquid scintillation counting of these species separated by using reverse-phase HPLC. Data were fitted to a single exponential, with yielding rate constants of 2.0 ± 0.1 s−1 for [3H]AcLys-14 H3 peptide deacetylation and 2.3 ± 0.2 s−1 for [3H]acetate adduct formation.