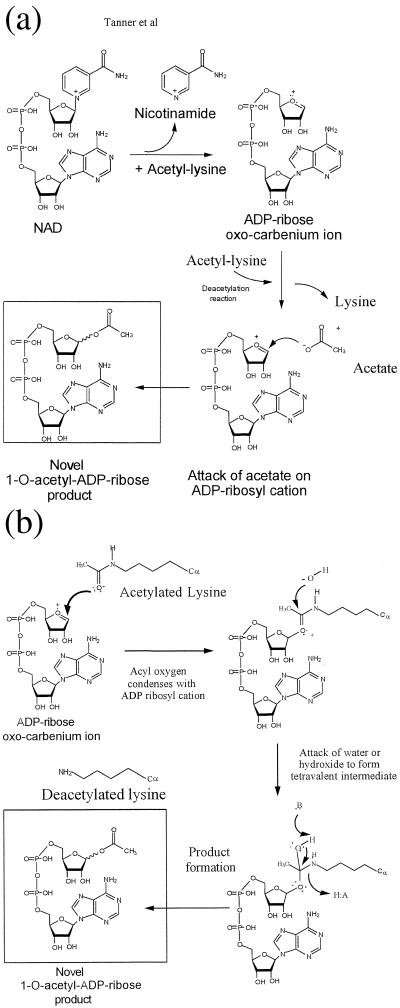
Figure 4.
Proposed catalytic mechanisms for the production of 1-O-acetyl-ADP-ribose. (A) Attack of transiently formed acetate on an oxocarbenium ADP-ribose intermediate. Nicotinamide elimination from NAD+ to produce an oxocarbenium ADP-ribose intermediate is coupled to acetyl-lysine binding or hydrolysis. The enzyme-bound acetate generated in the deacetylation reaction attacks the oxocarbenium cation to produce 1-O-acetyl-ADP-ribose. (B) Acetyl-lysine condenses directly with the oxocarbenium cation. After the formation of the oxocarbenium cation as in A, the acyl oxygen of acetyl-lysine condenses with the oxocarbenium cation. A hydroxide ion then attacks this intermediate to form a tetravalent intermediate, which can collapse to produce 1-O-acetyl-ADP-ribose through the use of enzyme general acid/base catalysis. With either mechanism (A or B), the chemistry could occur in either stepwise or concerted fashion. For clarity, we have drawn the chemical events as stepwise events.