Abstract
Peptoids (N-substituted polyglycines) represent a class of bioinspired oligomers that have unique physical and structural properties. Here we report the construction of “extended peptoids” based on aromatic building blocks, in which the N-alkylaminoacetyl group of the peptoid backbone has been replaced by an N-alkylaminomethylbenzoyl spacer. Both meta- and para-bromomethylbenzoic acids were synthesized, providing access to a new class of peptoids. Further, inclusion of hydrophilic side chains confers water solubility to these compounds, showing that, like simple peptoids, extended peptoids add an extra dimension to synthetic polyamide oligomers with potential application in a variety of biological contexts.
Keywords: peptoids, N-alkyl glycines, oligomers, bioinspired polymers
Peptoids1 (N-substituted glycines) represent an interesting class of biomimetic oligomers that are structurally related to peptides but have different biological2,3 and conformational4–6 properties. The submonomer method of peptoid synthesis introduced by Zuckermann, et al.,7 allows for considerable diversity to be incorporated into the construction of peptoid libraries due to the large number of commercially available primary amines. The submonomer method has been applied extensively in the creation of peptoid libraries8–12, and it has shown great versatility in incorporating a diverse set of amines13, 14 and hydrazines15. The peptoid scaffold has been further modified to create ureapeptoids16, 17 and incorporate a variety of chemoselective functionalities18. The bulk of these studies, however, have preserved the haloacetic acid component while focusing on variations in the nucleophilic displacement step. Here we report the solid-phase synthesis of a new class of peptoid-inspired biomimetics in which the haloacetic acid groups are replaced by either meta- or para-halomethylbenzoic acids (Figure 1b). These extended peptoids add further geometric diversity to oligomers based on the peptoid concept.
Figure 1.
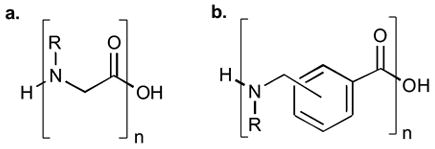
a. General peptoid structure. b. General extended peptoid structure
The synthesis of meta-extended peptoids is shown in Scheme 1. m- and p-bromomethylbenzoic acids were readily synthesized by bromination of the corresponding toluic acids19. All of the extended peptoids described here were synthesized on Wang resin (100–200 mesh, 1.1 mmole/g)2. The first bromomethylbenzoic acid submonomer was coupled to the resin with diisopropylcarbodiimide (DIC) and 4-dimethylaminopyridine (DMAP) in dry DCM over one hour at room temperature. The addition of subsequent bromomethylbenzoic acid submonomers was performed without DMAP. Primary amines (5 equivalents) were added in dry DMF and allowed to react at room temperature for one hour. Elongation of the extended peptoid chain was performed by repeating the above steps over several cycles, followed by cleavage from the resin with trifluoroacetic acid (TFA) containing triisopropylsilane.
Scheme 1.
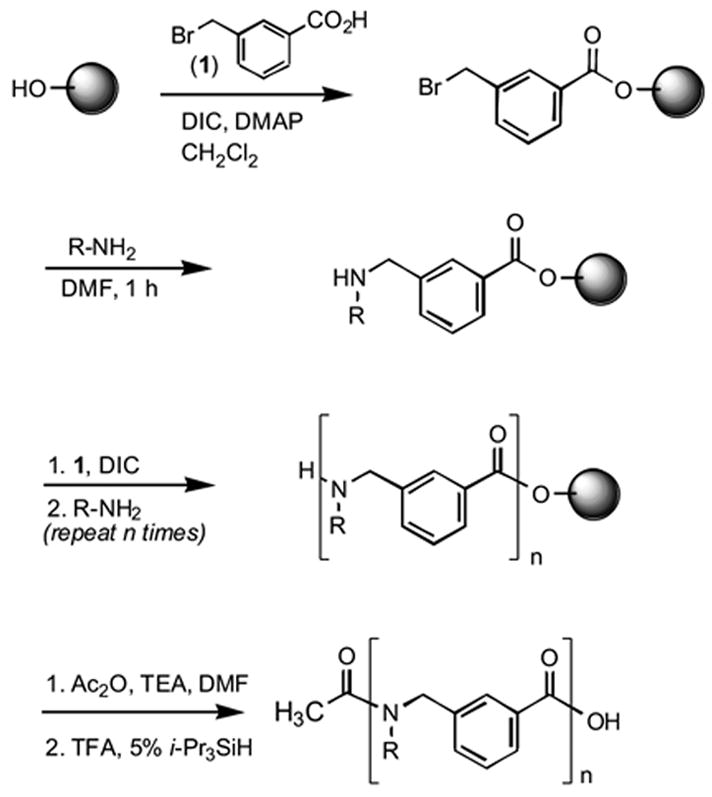
General scheme for the solid-phase synthesis of meta extended peptoids.
In order to establish the generality of the synthesis toward different primary amines, extended peptoid dimers were synthesized using amines 2 – 9. Amine coupling and acylation efficiencies were assessed using model compounds with the test amine at the first monomer position and amine 9 in the second position (Scheme 2). Amines with a primary substituent coupled efficiently, whereas those with a secondary substituent (e.g. 5 and 6) showed little to no reaction with the resin-bound bromomethyl benzoate ester (Table 1). Relatively electron deficient amines such as 2 and 8 also showed diminished coupling efficiencies at the amine addition step. This observation contrasts with reports in the literature on the efficient synthesis of similarly substituted peptoids. LC/MS analysis showed that the principal impurity among the poorly coupled amines was an end-capped deletion sequence, consistent with a failure at the nucleophilic displacement step. Despite these limitations, however, the high coupling efficiencies for amines 3, 4, 7, and 9 show that a range of functionalities can be tolerated during the amine addition step and that extended peptoids can be generated from a diverse collection of building blocks.
Scheme 2.
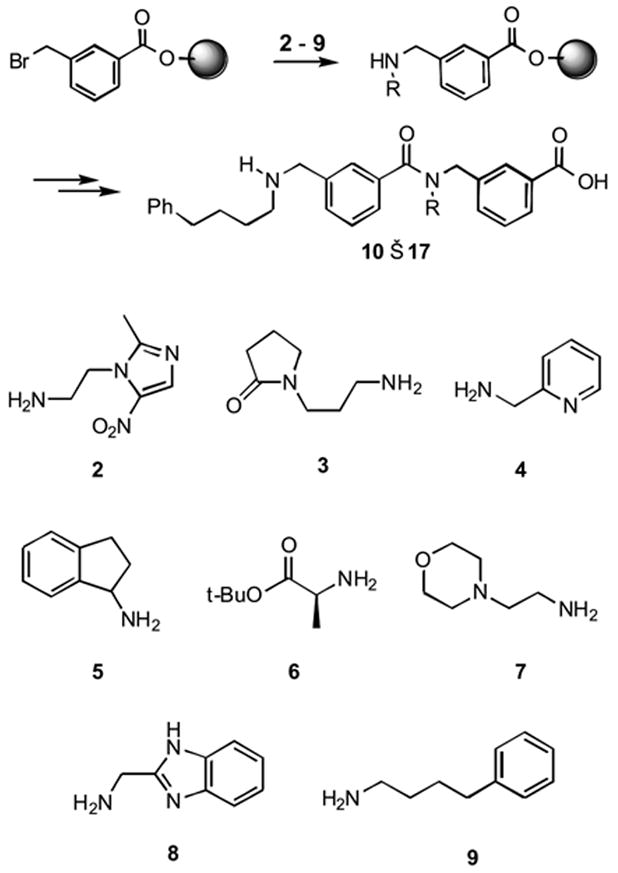
Monomer assessment studies using amines 2 – 9 on model extended peptoid backbone.
Table 1.
Coupling efficiencies of amines 2 – 9
| amine | compound | %puritya | %crude yieldc |
|---|---|---|---|
| 2 | 10 | 9 | 54 |
| 3 | 11 | 87 | >99 |
| 4 | 12 | 79 | >99 |
| 5 | 13 | 0b | 0b |
| 6 | 14 | 0b | 0b |
| 7 | 15 | >99 | >99 |
| 8 | 16 | 46 | 55 |
| 9 | 17 | >99 | >99 |
Determined by % area of light scattering signal in LC/MS analysis of crude product after cleavage from resin.
No product was detected by mass.
TFA removed by evaporation; crude product washed several times with Et2O, resuspended in 1:1 H2O ACN and lyophilized.
To demonstrate the viability of synthesizing larger extended peptoids, pentamers using both para and meta bromotoluic acids and amines 7 and 9 were made. Product purities after each monomer addition were assessed by HPLC and yields were determined by weighing the final cleaved products. Excellent purities and moderate isolated, purified yields were obtained (see Table 2.) NMR spectra taken at room temperature for all four synthesized pentamers were highly complex, most likely due to the presence of multiple amide bond rotamers.
Table 2.
Yields and purities of extended peptoid oligomers
| spacer | sequencea | %purityb | %purified yieldc | |
|---|---|---|---|---|
| 18 | m | Ac-7-7-7-7 | 94 | 37 |
| 19 | m | Ac-9-9-9-9-9 | 99 | 12 |
| 20 | p | Ac-7-7-7-7 | 95 | 20 |
| 21 | p | Ac-9-9-9-9-9 | 99 | 12 |
Ac = N-acyl.
Determined by % area of light scattering signal in LC/MS analysis of crude product after cleavage from resin.
Compounds purified by preparative HPLC and lyophilized.
When NMR spectra were obtained at 100°C in DMSO-d6, the spectra for pentamers based on amine 9 were greatly simplified and amenable to straightforward interpretation; however, pentamers based on amine 7 degraded (in both DMSO-d6 and D2O) at the high temperatures required to coalesce the amide rotamers3. Pentamers incorporating amine 7 were found to be highly water-soluble (80 mg/ml), suggesting that the backbone’s intrinsic hydrophobicity can be overcome with appropriate hydrophilic side chains to generate water-soluble extended peptoids.
The discrepancy between the high purity of the crude product and moderate isolated yield is likely due to aminolysis of the peptide from the Wang linker. Future studies will be aimed at optimizing conditions on the Rink resin or attempting syntheses on the more sterically hindered 2-chlorotrityl resin. Microwave-based synthesis of these compounds was explored but showed no significant improvement for either impurities or yields (data not shown).
In principle, the extended peptoid concept could be further elaborated to include vinylogous, heterocyclic and polycyclic spacer elements that contain reactive allylic and benzylic halides, further increasing the potential diversity of this class of compounds. These compounds represent a new type of oligomer based on a simple elaboration of standard peptoid chemistry, providing access to a large variety of new compounds with potentially interesting properties. Among these properties, the inherent hydrophobicity and rigidity of these extended peptoids, in particular, may be well suited as macromolecule ligands for proteomics and drug discovery applications.
Supplementary Material
Acknowledgments
We are grateful to the following agencies for financial support of this work: The California Institute for Bioengineering, Biotechnology and Quantitative Biomedical Research (QB3) opportunity fund; the National Cancer Institute (1R01CA104569-01); California Cancer Research Coordinating Committee (Award SC-04-76). We thank Mr. J. Loo for assistance with the NMR experiments. We are also grateful to Roche Palo Alto for the donation of the mass spectrometer used for this work.
Footnotes
Attempts to perform the synthesis on Rink amide resin resulted in significant impurities corresponding to nucleophilic attack by the Rink resin amine onto the bromomethyl group.
It should be noted that the aromatic resonances due to the side chain of 7 were not distinguishable from those of the extended peptoid backbone in the NMR spectrum.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Marlowe CK, Spellmeyer DC, Tan R, Frankel AD, Santi DV, Cohen FE, Bartlett PA. Proc Natl Acad Sci USA. 1992;89:9367–71. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen JT, Porter M, Amoui M, Miller WT, Zuckermann RN, Lim WA. Chem Biol. 2000;7:463–73. doi: 10.1016/s1074-5521(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 3.Zuckermann RN, Martin EJ, Spellmeyer DC, Stauber GB, Shoemaker KR, Kerr JM, Figliozzi GM, Goff DA, Siani MA, Simon RJ, Banville SC, Brown EG, Wang L, Richter LS, Moos WH. J Med Chem. 1994;37:2678–85. doi: 10.1021/jm00043a007. [DOI] [PubMed] [Google Scholar]
- 4.Kirshenbaum K, Barron AE, Goldsmith RA, Armand P, Bradley EK, Truong KT, Dill KA, Cohen FE, Zuckermann RN. Proc Natl Acad Sci USA. 1998;95:4303–8. doi: 10.1073/pnas.95.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armand P, Kirshenbaum K, Falicov A, Dunbrack RL, Jr, Dill KA, Zuckermann RN, Cohen FE. Fold Des. 1997;2:369–75. doi: 10.1016/S1359-0278(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 6.Sanborn TJ, Wu CW, Zuckermann RN, Barron AE. Biopolymers. 2002;63:12–20. doi: 10.1002/bip.1058. [DOI] [PubMed] [Google Scholar]
- 7.Figliozzi GM, Goldsmith R, Ng SC, Banville SC, Zuckermann RN. Meth Enzymol. 1996;267:437–47. doi: 10.1016/s0076-6879(96)67027-x. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JE, Uno T, Hamer JD, Cohen FE, Dwarki V, Zuckermann RN. A combinatorial approach to the discovery of efficient cationic peptoid reagents for gene delivery. Proc Natl Acad Sci USA. 1998;95:1517–22. doi: 10.1073/pnas.95.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boden P, Eden JM, Hodgson J, Horwell DC, Hughes J, McKnight AT, Lewthwaite RA, Pritchard MC, Raphy J, Meecham K, Ratcliffe GS. J Med Chem. 1996;39:1664–75. doi: 10.1021/jm950892r. [DOI] [PubMed] [Google Scholar]
- 10.Linusson A, Wold S, Norden B. Mol Divers. 1998;4:103–14. doi: 10.1023/a:1026416430656. [DOI] [PubMed] [Google Scholar]
- 11.Humet M, Carbonell T, Masip I, Sanchez-Baeza F, Mora P, Canton E, Gobernado M, Abad C, Perez-Paya E, Messeguer A. J Comb Chem. 2003;5:597–605. doi: 10.1021/cc020075u. [DOI] [PubMed] [Google Scholar]
- 12.Alluri PG, Reddy MM, Bachhawat-Sikder K, Olivos HJ, Kodadek T. J Am Chem Soc. 2003;125:13995–4004. doi: 10.1021/ja036417x. [DOI] [PubMed] [Google Scholar]
- 13.Uno T, Beausoleil E, Goldsmith RA, Levine BH, Zuckermann RN. Tet Lett. 1999;40:1475–1478. [Google Scholar]
- 14.Burkoth TS, Fafarman AT, Charych DH, Connolly MD, Zuckermann RN. J Am Chem Soc. 2003;125:8841–5. doi: 10.1021/ja0352101. [DOI] [PubMed] [Google Scholar]
- 15.Cheguillaume A, Lehardy F, Bouget K, Baudy-Floc’h M, Le Grel P. J Org Chem. 1999;64:2924–2927. doi: 10.1021/jo981487l. [DOI] [PubMed] [Google Scholar]
- 16.Wilson ME, Nowick JS. Tet Lett. 1998;39:6613–6616. [Google Scholar]
- 17.Kruijtzer JAW, Lefeber DJ, Liskamp RMJ. Tet Lett. 1997;38:5335–5338. [Google Scholar]
- 18.Horn T, Lee BC, Dill KA, Zuckermann RN. Bioconjug Chem. 2004;15:428–35. doi: 10.1021/bc0341831. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi D, Sakaguchi S, Ishii Y. J Org Chem. 1998;63:6023–6026. doi: 10.1021/jo972263q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


