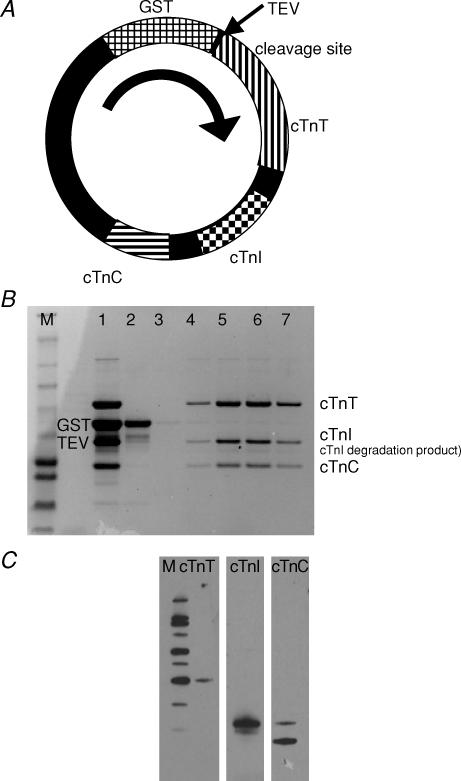
Figure 1. Coexpression of recombinant human cardiac troponin (rhcTn) complex.
A, cDNA sequences for all three subunits of hcTn were cloned into pET 41a+ along with promotors and terminators for coexpression in E. coli. The plasmid was engineered with a GST tag at the N-terminus of hcTnT, and modified to include a TEV protease site between GST and hcTnT. B, SDS-PAGE analysis of the coexpressed rhcTn complex demonstrates that the three subunits form a complex; densitometry analysis indicates ∼96% purity. M, marker; 1, rhcTn complex after GST affinity purification and TEV protease cleavage; 2, anion exchange chromatography (DE52) void volume, containing GST tag and TEV protease; and 3–7, anion exchange chromatography fractions eluted by salt gradient. C, Western blot analysis of the coexpressed rhcTn complex. Specific antibodies for hcTnT (left), hcTnI (centre), and hcTnC (right) detected bands of the appropriate sizes for each subunit (see Methods). M, marker (MagicMark XP Western Protein Standard; Invitrogen).