Abstract
In hypertension, exercise elicits excessive elevations in mean arterial pressure (MAP) and heart rate (HR) increasing the risk for adverse cardiac events and stroke during physical activity. The exercise pressor reflex (a neural drive originating in skeletal muscle), central command (a neural drive originating in cortical brain centres) and the tonically active arterial baroreflex contribute importantly to cardiovascular control during exercise. Each of these inputs potentially mediates the heightened cardiovascular response to physical activity in hypertension. However, given that exercise pressor reflex overactivity is known to elicit enhanced circulatory responses to exercise in disease states closely related to hypertension (e.g. heart failure), we tested the hypothesis that the exaggerated cardiovascular response to exercise in hypertension is mediated by an overactive exercise pressor reflex. To test this hypothesis, we used a rat model of exercise recently developed in our laboratory that selectively stimulates the exercise pressor reflex independent of central command and/or the arterial baroreflex. Activation of the exercise pressor reflex during electrically induced static muscle contraction in the absence of input from central command resulted in significantly larger increases in MAP and HR in male spontaneously hypertensive rats as compared to normotensive Wistar-Kyoto rats over a wide range of exercise intensities. Similar findings were obtained in animals in which input from both central command and the arterial baroreflex were eliminated. These findings suggest that the enhanced cardiovascular response to exercise in hypertension is mediated by an overactive exercise pressor reflex. Potentially, effective treatment of exercise pressor reflex dysfunction may reduce the cardiovascular risks associated with exercise in hypertension.
Hypertension is a major risk factor for the development of a number of cardiovascular disorders including coronary heart disease, peripheral arterial disease, stroke, renal disease and heart failure (Pescatello et al. 2004). Exercise programmes that include both dynamic (Fagard, 2001; Kelley et al. 2001) and static (Kiveloff & Huber, 1971; Kelley & Kelley, 2000) forms of physical activity have been shown to improve cardiovascular health and lower resting blood pressure in hypertensive patients. Unfortunately, in hypertensive individuals, exercise produces excessive increases in arterial blood pressure (BP), from a chronically elevated baseline, and heart rate (HR) (Pickering, 1987; Kahn, 1991; Kazatani et al. 1995). These dangerous elevations in circulatory haemodynamics may increase the risk for cardiac events such as acute myocardial infarction or cardiac arrest as well as stroke during physical activity (Hoberg et al. 1990; Mittleman et al. 1993; Kokkinos et al. 2002) limiting the safety of the prescription of exercise. Therefore, determining the cause of this cardiovascular hyperexcitability during acute bouts of exercise is very important.
Currently, the neurophysiological mechanisms driving the excessive increases in BP and HR during exercise in hypertension are largely unknown. Two distinct neural control mechanisms activated during exercise, central command and the exercise pressor reflex, are known to contribute significantly to autonomic circulatory adjustments during physical activity. Central command is a feedforward neural drive originating in higher brain centres that is associated with the volitional component of exercise (Krogh & Lindhard, 1913; Goodwin et al. 1972). Central command contributes to elevations in BP and HR during exercise via withdrawal of parasympathetic nerve activity and increases in sympathetic nerve activity. The exercise pressor reflex is a feedback peripheral neural drive originating in active skeletal muscle (McCloskey & Mitchell, 1972; Kaufman et al. 1983). Stimulation of this reflex during muscle contraction induces haemodynamic changes predominately via activation of the sympathetic nervous system. In addition to these neural inputs, the cardiovascular response to exercise is modulated by the tonically active arterial baroreflex. The afferent fibres of the arterial baroreflex originate in the carotid sinus and aortic arch and serve to regulate blood pressure on a beat-to-beat basis by continually adjusting HR, stroke volume and peripheral resistance at rest and during exercise (Mancia & Mark, 1983). Given these essential functions, central command, the exercise pressor reflex and/or the arterial baroreflex potentially mediate the heightened cardiovascular responses to exercise in hypertension.
Of these neural inputs, the exercise pressor reflex has previously been shown to mediate enhanced cardiovascular responses to exercise in disease states closely associated with chronic high blood pressure (e.g. heart failure) (Middlekauff et al. 2000; Smith et al. 2003a, 2005a, b; Li et al. 2004; Ansorge et al. 2005) Therefore, we hypothesized that the exaggerated cardiovascular response to physical activity in hypertension is mediated by an overactive exercise pressor reflex independent of central command and the arterial baroreflex. To test this hypothesis, we selectively stimulated the exercise pressor reflex in normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats using an exercise model recently developed in our laboratory (Smith et al. 2001). In this decerebrate rat model of exercise, electrical stimulation of spinal ventral roots innervating hindlimb skeletal muscle evokes static muscle contractions. These contractions elicit increases in BP and HR that have been shown to be mediated by the exercise pressor reflex (Smith et al. 2001). It is important to note that as muscle contraction is induced involuntarily, central command is not activated in this preparation. Further, input from central command is completely abolished as the decerebration procedure removes the areas in the cerebral cortex from which this input originates. In addition, exercise pressor reflex function can be evaluated in this model independently from the arterial baroreflex by baro-denervating animals.
Using this model, we have determined that exercise pressor reflex activity is potentiated in hypertensive rats and mediates abnormal augmentations in both BP and HR during muscle contraction independent of input from central command and the arterial baroreflex. The use of this model allows further dissection of the pathophysiology of hypertension and may lead to the development of novel therapeutic strategies targeted at improving circulatory haemodynamics during physical activity. Effective treatment of dysfunctional neural cardiovascular control in hypertension could reduce the risks associated with exercise in this disease.
Methods
Experiments were performed in age-matched (14–20 weeks) male WKY (n = 24) and SHR (n = 27) rats (Harlan). The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. All studies were conducted in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preliminary measurements: echocardiography
In order to determine the effects of chronic hypertension on cardiac performance in the animal population used in this study, left ventricular function was assessed using echocardiography. Two weeks prior to acute physiological experimentation, anaesthesia was induced with isoflurane gas anaesthesia (1–2% in 100% oxygen) and transthoracic echocardiography (Vivid 7 Pro, GE Medical Systems) was performed. Echocardiographic M-mode images of the left ventricle were obtained using a parasternal short-axis view. Left ventricular end diastolic (LVEDD) and systolic (LVESD) dimensions were measured and used to calculate the percentage of left ventricular fractional shortening (FS), an index of left ventricular function, for each animal using the following equation: FS = (LVEDD – LVESD)/LVEDD × 100.
Acute surgical procedures
General procedures
As previously described (Smith et al. 2001), rats were initially anaesthetized with isoflurane gas (2–4% in 100% oxygen) and intubated for mechanical ventilation. Levels of inhalant gas were increased as indicated by a withdrawal reflex to pinching of the hindpaw, presence of a corneal reflex and/or spontaneous increases in HR. Fluid-filled catheters were placed within the right jugular vein for the administration of experimental drugs and the left common carotid artery for the measurement of arterial blood pressure. A continuous infusion (2 ml 1 m NaHCO3 and 10 ml 5% dextrose in 38 ml Ringer solution) was established via the jugular vein (3–5 ml h−1 kg−1) to stabilize fluid balance and maintain baseline arterial blood pressure (Quintin et al. 1989). Dexamethasone (0.2 mg) was given intramuscularly to minimize oedema (Tian & Duffin, 1996). Arterial blood gases and pH were measured periodically using an automated blood gas analyser (Model ABL 5, Radiometer). Blood gases were maintained within normal ranges (arterial PO2, > 80 mmHg; arterial PCO2, 35–45 mmHg; pH 7.3–7.4) throughout the experiment. Body temperature was maintained between 36.5°C and 38.0°C by a customized heating-controlled pad. A laminectomy exposing the lower lumbar portions of the spinal cord (L2–L6) was performed. The meningial layers surrounding the cord were cut and reflected. The L4 and L5 ventral roots were carefully isolated and sectioned. The cut peripheral ends of the roots were placed on insulated bipolar platinum electrodes. The exposed neural tissue was immersed in mineral oil. The calcaneal bone of the right hindlimb was cut and the Achilles' tendon connected to a force transducer for the measurement of muscle tension. Subsequently, animals were decerebrated and rendered insentient by sectioning the brain rostral to the superior colliculus and aspirating the forebrain. To minimize cerebral haemorrhage, the right common carotid artery was ligated prior to decerebration and small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the exposed surfaces of the brain. In addition, cotton balls were used to pack the cranial cavity. After aspirating the forebrain, gas anaesthesia was discontinued. A minimum recovery period of 1 h was employed after decerebration before beginning any experimental protocol. This allowed sufficient time for the effects of isoflurane anaesthesia to completely dissipate. Animals were placed in a stereotaxic head unit (Kopf Instruments) and customized spinal frame throughout the remainder of the experiment.
Baro-denervation procedures
In a subset of animals, baro-denervation procedures were performed before decerebration. Sino-aortic denervation (SAD) was achieved using the method originally described by Krieger (1964). A midline incision was made in the ventral region of the neck and the common carotid artery and carotid bifurcation exposed. The superior cervical ganglion was removed, the aortic depressor nerve cut, and the superior laryngeal nerve sectioned at its junction with the vagus nerve to eliminate input from aortic baroreceptor afferent fibres. The common carotid artery, carotid bifurcation, and internal and external carotid arteries were carefully stripped of neural and connective tissues and painted with 10% phenol in 70% ethanol to ablate baroreceptor afferent fibres in the carotid sinus region. Previous experiments have shown that this method of dissection and treatment of the carotid sinus ensures complete denervation (Krieger, 1963).
Experimental protocols
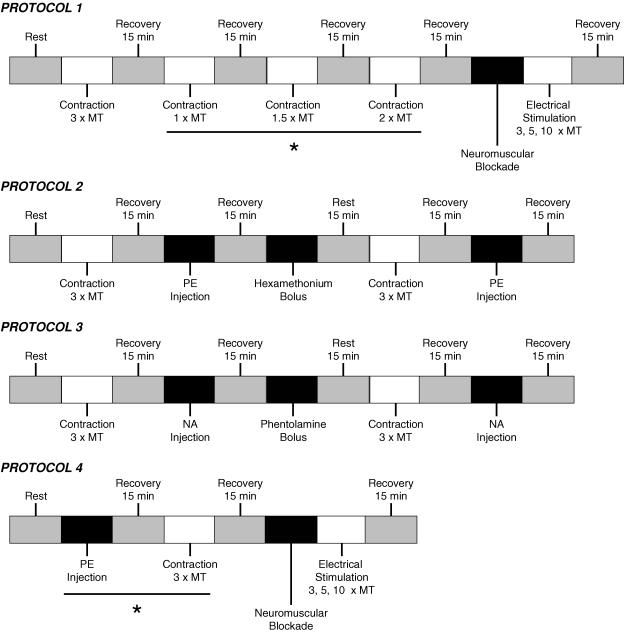
A schematic representation of each protocol is presented in Fig. 1.
Figure 1. Schematic representation of protocols used.
MT, motor threshold; PE, phenylephrine; NA, noradrenaline. *Trials were randomized.
Protocol 1
To activate the exercise pressor reflex in WKY (n = 14) and SHR (n = 18) animals, the gastrocnemius and soleus muscles of the right hindlimb were contracted by electrically stimulating the L4 and L5 ventral roots for 30 s. Constant current stimulation was used at 3 times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. These stimulus parameters are known to generate maximal tension development in rats (Smith et al. 2001). As stated previously, these procedures have been shown to elicit increases in BP and HR that are due to the selective activation of the exercise pressor reflex independent of input from central command in this animal model (Smith et al. 2001). In a subset of WKY (n = 10) and SHR (n = 9) rats, additional muscle contractions were induced using randomized submaximal stimulus intensities (i.e. 1–2 times motor threshold, 0.1 ms pulse duration, 40 Hz). Prior to all contractions, the muscles were preloaded with 70–100 g of tension. At the conclusion of each experiment, the neuromuscular blocking agent vecuronium bromide (1 mg ml−1) was administered intravenously and stimulation of the ventral roots was repeated at 3, 5 and 10 times motor threshold. This manoeuvre was employed to validate that the cardiovascular responses elicited resulted from muscle contraction rather than by the inadvertent activation of sensory afferent fibres during electrical stimulation.
It should be noted that activation of the exercise pressor reflex during muscle contraction in this rat model has previously been shown to increase both mean arterial pressure (MAP) and HR (Smith et al. 2003a). Importantly, sectioning the spinal sensory dorsal roots innervating the contracting skeletal muscle abolishes the cardiovascular response to contraction in this preparation (Smith et al. 2001). This finding indicates that a neural reflex originating in skeletal muscle (i.e. the exercise pressor reflex) mediates the resultant cardiovascular responses.
Protocol 2
The exercise pressor reflex induces increases in HR and MAP predominately via increased activation of the sympathetic nervous system. We reasoned therefore that the cardiovascular responses to muscle contraction should be abolished by sympathetic blockade if mediated by the exercise pressor reflex. To test this idea, the cardiovascular response to activation of the exercise pressor reflex was assessed before and after sympathetic blockade in a subset of WKY (n = 4) and SHR (n = 4) animals. In both groups, maximal static contraction of the gastrocnemius and soleus muscles was induced as described in protocol 1. Subsequently, the ganglionic blocking agent hexamethonium (30 mg kg−1) was administered intravenously and the contraction manoeuvre repeated. In addition, to determine the effectiveness of ganglionic blockade, the HR and BP response to an intravenous bolus of the pressor agent phenylephrine (2 mg kg−1) was assessed before and after hexamethonium administration. Reductions in the HR response to phenylephrine-induced elevations in BP after administration of hexamethonium verify the completeness of ganglionic blockade. The concentrations of hexamethonium and phenylephrine administered have been shown previously to be effective in this animal model (Radaelli et al. 1998).
Protocol 3
As a corollary to protocol 2, activation of the exercise pressor reflex during muscle contraction was induced before and after sympathetic blockade with the α-adrenergic blocking agent phentolamine (5 mg kg−1) in additional SHR animals (n = 4). To determine the effectiveness of sympathetic blockade, the BP response to an intravenous bolus of noradrenaline (5 μg kg−1) was given before and after phentolamine. As noradrenaline is an α-receptor agonist, reductions in the BP response to noradrenaline administration after phentolamine verify the completeness of sympathetic blockade. The concentrations of phentolamine and noradrenaline administered have previously been shown to be effective in this animal model (Muntzel et al. 1997).
Protocol 4
The arterial baroreflex modulates the cardiovascular response to exercise (Mancia & Mark, 1983). In order to assess exercise pressor reflex function in the absence of both central command and arterial baroreflex input, a subset of WKY (n = 7) and SHR (n = 6) animals were additionally baro-denervated. To confirm the completeness of the sino-aortic baro-denervation procedure, the baroreflex was challenged by an intravenous bolus injection of the pressor agent phenylephrine (2 mg kg−1). As a control, baroreflex function was likewise assessed during a phenylephrine challenge in baro-intact WKY (n = 6) and SHR (n = 6) animals. Subsequently, maximal static contraction of the gastrocnemius and soleus muscles were electrically induced as described in protocol 1. At the conclusion of each experiment, the neuromuscular blocking agent vecuronium bromide (1 mg ml−1) was administered intravenously and the ventral roots stimulated at 3, 5 and 10 times motor threshold.
Data acquisition
Arterial blood pressure was measured by connecting the left arterial catheter to a pressure transducer (Model DTX plus-DT, NN12, Ohmeda). MAP was obtained by integrating the arterial pressure signal with a time constant of 1–4 s. HR was derived from the blood pressure pulse wave using a biotachometer (Gould Instruments). Hindlimb tension was quantified using a force transducer (FT-10, Grass Instruments). Baseline values for all variables were determined by analysing 30 s of data immediately prior to muscle contraction or intravenous drug delivery. The peak response of each variable was defined as the greatest change from baseline elicited by the experimental stimulus. All cardiovascular and contractile force data were acquired, recorded and analysed using hardware and software for the CED micro 1401 system (Cambridge Electronic Design).
Morphological measurements
At the conclusion of experimentation, animals were killed by intravenous administration of sodium pentobarbital (120 mg kg−1). In all animals, the heart was excised and weighed. Additionally, the lungs and tibia were harvested, weighed and measured.
Statistical analyses
On all data sets, statistics were performed using correlation and regression analyses, Student t tests or ANOVA with Student–Newman–Kuels post hoc tests employed as appropriate. The significance level was set at P < 0.05. Results are presented as means ± s.e.m. Statistical analyses were conducted using SigmaStat software (Jandel Scientific Software, SPSS Inc.).
Results
Characterization of hypertensive model
Morphometric and baseline haemodynamic data for WKY and SHR animals are presented in Table 1. The body weights of WKY rats were not different from SHR rats. Ratios of heart weight to body weight and heart weight to tibial length were significantly greater in SHR than WKY rats. In contrast, the lung weight to body weight ratio was not different between the groups. As expected, baseline MAP was markedly greater in SHR than WKY rats.
Table 1.
Morphometric characteristics and baseline haemodynamics
| WKY | SHR | |
|---|---|---|
| n | 24 | 27 |
| Body weight (g) | 351 ± 7 | 337 ± 6 |
| HR (beats min−1) | 393 ± 11 | 372 ± 16 |
| MAP (mmHg) | 89 ± 8 | 149 ± 5* |
| Heart weight/body weight (mg g−1) | 3.2 ± 0.1 | 3.7 ± 0.1* |
| Heart weight/tibial length (mg mm−1) | 29.1 ± 1.0 | 34.3 ± 1.5* |
| Lung weight/body weight (mg g−1) | 6.7 ± 0.5 | 7.3 ± 0.4 |
Values are means ±s.e.m.
P < 0.05 compared to WKY rats.
Echocardiographic assessment of left ventricular function
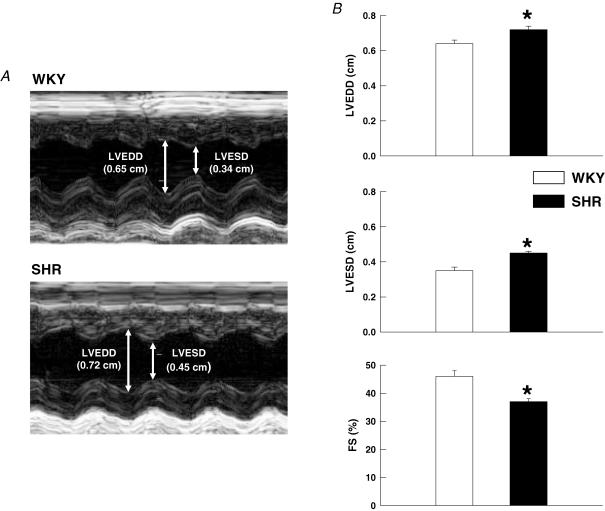
As determined by transthoracic echocardiography (Fig. 2A), SHR rats exhibited a moderate level of left ventricular dysfunction as compared to WKY animals. As presented in Fig. 2B, left ventricular FS was significantly reduced in SHR (37 ± 1%) as compared to WKY (46 ± 2%) rats. Commensurate with this finding, both LVEDD and LVESD were greater in SHR (0.72 ± 0.02 and 0.45 ± 0.01 cm, respectively) than in WKY animals (0.64 ± 0.02 and 0.34 ± 0.02 cm, respectively).
Figure 2. Echocardiographic assessment of left ventricular function.
A, M-mode echocardiographic images from representative WKY and SHR animals. Arrows demarcate LVEDD and LVESD dimensions. B, echocardiographic analysis determined that both left ventricular end diastolic (LVEDD) and systolic (LVESD) dimensions were significantly greater in SHR animals. As a result, fractional shortening (FS) was reduced in SHR as compared to WKY rats. *P < 0.05 compared to WKY rats.
The exercise pressor reflex is overactive in hypertension
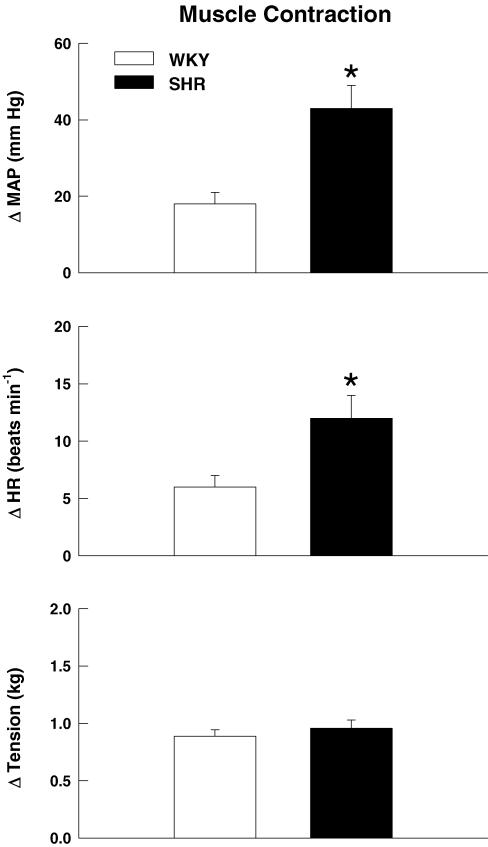
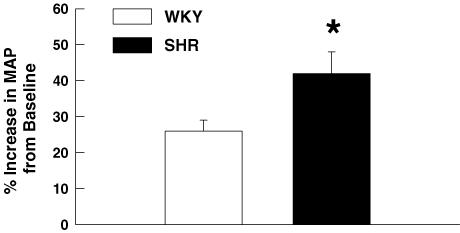
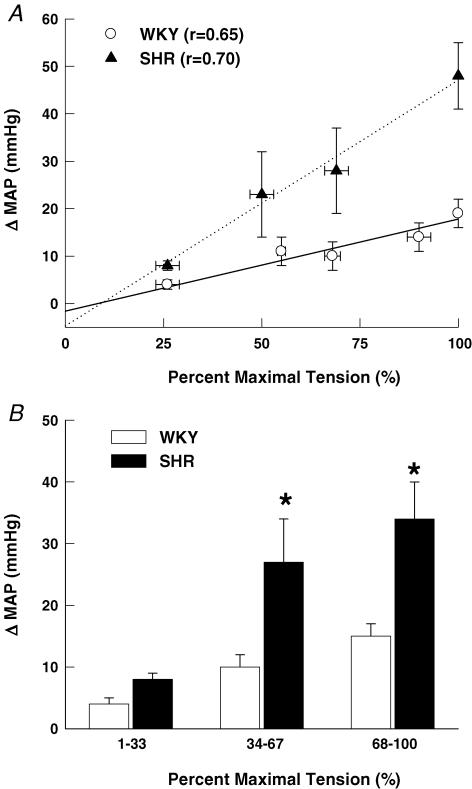
At maximal intensities, activation of the exercise pressor reflex during electrically induced static muscle contraction (Fig. 3) elicited significantly larger elevations in MAP and HR in SHR as compared to WKY rats. As baseline MAP was significantly greater in SHR than WKY, the pressor response to activation of the exercise pressor reflex was also quantified as a percentage of baseline MAP (Fig. 4). Despite the marked elevation in baseline blood pressure in SHR rats, the percentage increase in MAP from baseline in response to activation of the exercise pressor reflex remained significantly greater in SHR (42 ± 7%) than WKY (26 ± 3%) animals. Moreover, the MAP response to activation of the exercise pressor reflex was positively correlated (r = 0.74, P < 0.001) to baseline blood pressure such that the greater the basal MAP the larger the pressor response to static muscle contraction. As presented in Fig. 5A, graded contractions at submaximal work intensities elicited pressor responses that were positively correlated to tension development in both WKY (r = 0.65; P < 0.001) and SHR rats (r = 0.70; P < 0.001). Linear regression analyses determined that the slope of this relationship was significantly larger in SHR (0.53 ± 0.09) than WKY rats (0.17 ± 0.03). As a result, the MAP responses to contraction were found to be augmented in SHR as compared to WKY rats over a wide range of work intensities. For comparison, data were binned into low (1–33% maximal tension), medium (34–67% maximal tension) and high (68–100% maximal tension) levels of contractile intensity (Fig. 5B). This analysis determined that the pressor response to muscle contraction was significantly greater in SHR as compared to WKY animals beginning at exercise intensities corresponding to 34–67% of maximal tension development. In all animals, neuromuscular blockade completely abolished the cardiovascular response to electrical stimulation of the L4 and L5 ventral roots at 3, 5 and 10 times motor threshold (data not shown). The latter finding indicates that the cardiovascular responses elicited by static muscle contraction were not due to direct electrical stimulation of muscle sensory afferent fibres but mediated, instead, by activation of the exercise pressor reflex.
Figure 3. Cardiovascular responses to activation of the exercise pressor reflex in WKY and SHR animals.
Contraction-induced increases in MAP and HR were significantly greater in SHR as compared to WKY rats at maximal levels of tension development. *P < 0.05 compared to WKY rats.
Figure 4. The blood pressure response to activation of the exercise pressor reflex in WKY and SHR rats.
When expressed as a percentage increase from baseline blood pressure, the MAP response to static muscle contraction was significantly larger in SHR than in WKY rats. *P < 0.05 compared to WKY rats.
Figure 5. The blood pressure response to muscle contraction at graded intensities.
A, static contractions at submaximal workloads elicited pressor responses that were positively correlated to force development in both WKY and SHR rats (P < 0.001). B, the contraction-induced increase in MAP was significantly larger in SHR than WKY rats beginning at intensity levels corresponding to 34–67% of maximal tension development. For correlation analysis, all data points within a group were used for statistical testing and averaged for presentation only. *P < 0.05 compared to WKY rats.
The exercise pressor reflex mediates the enhanced blood pressure response to muscle contraction in hypertensive rats: effects of sympathetic blockade
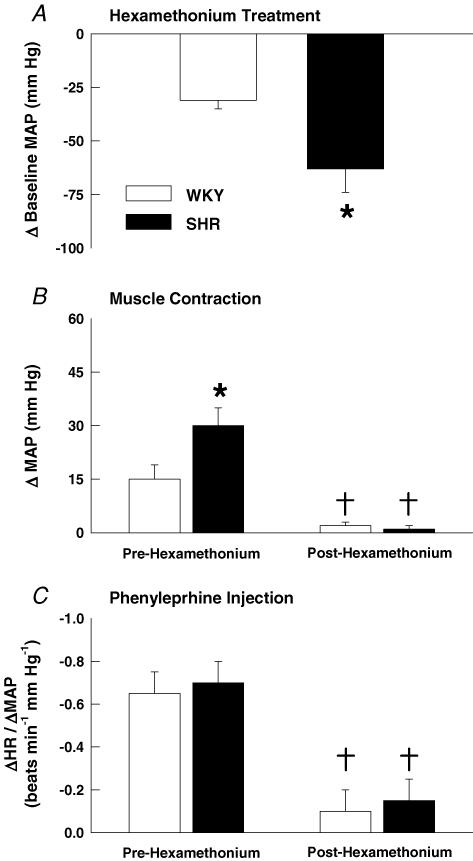
Using ganglionic blockade to inhibit sympathetic nerve activity, the pressor response to static muscle contraction was examined before and after the administration of hexamethonium. Ganglionic blockade significantly reduced baseline MAP by 31 ± 4 mmHg in WKY and 63 ± 11 mmHg in SHR rats (Fig. 6A). Likewise, baseline HR was reduced by 61 ± 7 beats min−1 in WKY and 74 ± 12 beats min−1 in SHR rats. The larger decrease in MAP in hypertensive rats following ganglionic blockade is indicative of elevations in basal sympathetic drive. Complementing this finding, the MAP response to muscle contraction prior to hexamethonium treatment was significantly greater in SHR as compared to WKY rats (Fig. 6B). However, in both WKY and SHR rats, the pressor response to activation of the exercise pressor reflex by muscle contraction was essentially eliminated by ganglionic blockade (Fig. 6B). This finding confirms that the exercise pressor reflex elicited increases in blood pressure in both WKY and SHR rats via the sympathetic nervous system. In order to validate the completeness of ganglionic blockade during these experiments, phenylephrine was administered before and after hexamethonium. In WKY and SHR rats, significant reductions in HR were evoked in response to phenylephrine-induced increases in MAP. After hexamethonium treatment, the pressor response to phenylephrine was similar in each experimental group but the decrease in HR in response to this pressure change was significantly attenuated in both WKY and SHR animals. This was reflected by a significant reduction in the ratio of change in HR to change in MAP (ΔHR/ΔMAP) after ganglionic blockade in WKY and SHR rats (Fig. 6C).
Figure 6. Ganglionic blockade with hexamethonium in WKY and SHR animals.
A, hexamethonium treatment reduced baseline blood pressure in both WKY and SHR rats. The magnitude of this reduction was greater in SHR rats. B, hexamethonium treatment abolished the pressor response to static muscle contraction in both animal groups. C, in WKY and SHR rats, hexamethonium treatment significantly reduced the ratio between the decrease in HR and the increase in MAP evoked by phenylephrine administration validating the effectiveness of the ganglionic block. *P < 0.05 compared to WKY rats. †P < 0.05 compared to before administration of hexamethonium.
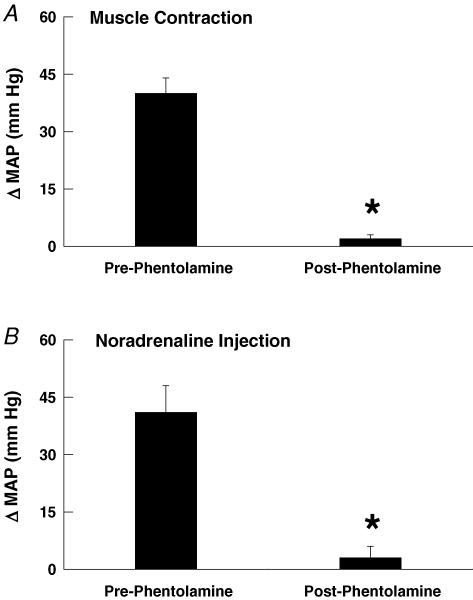
In addition to these experiments, we used phentolamine (an α-adrenergic antagonist) as a secondary index of sympathetic drive in a subset of SHR animals. In these rats, phentolamine induced a 71 ± 9 mmHg reduction in baseline MAP. Activation of the exercise pressor reflex by static muscle contraction induced an increase in MAP of 40 ± 3 mmHg before sympathetic blockade with phentolamine but only 2 ± 1 mmHg after phentolamine (Fig. 7A). This finding confirms that the exercise pressor reflex elicited the exaggerated increases in blood pressure via the sympathetic nervous system. In order to assess the completeness of the sympathetic block, noradrenaline was given before and after phentolamine administration. Noradrenaline raised MAP by 41 ± 7 mmHg before phentolamine but by only 3 ± 3 mmHg after administration of phentolamine (Fig. 7B).
Figure 7. Sympathetic blockade with phentolamine in SHR animals.
A, phentolamine treatment abolished the pressor response to activation of the exercise pressor reflex in SHR rats. B, phentolamine treatment abolished the pressor response evoked by intravenous injection of noradrenaline validating the effectiveness of the sympathetic block. *P < 0.05 compared to before administration of phentolamine.
The effects of baro-denervation on exercise pressor reflex function
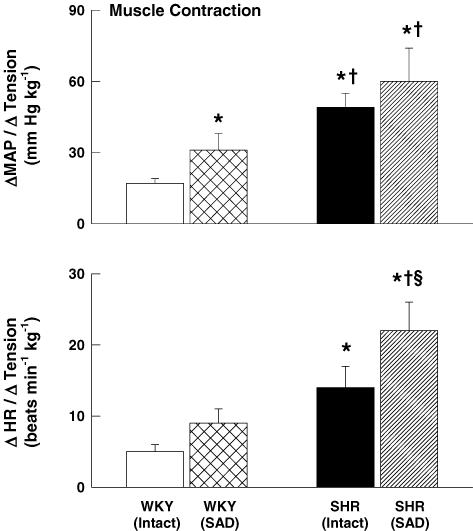
In order to assess the effect of arterial baroreflex input on exercise pressor reflex activity in normal and hypertensive rats, we selectively activated the exercise pressor reflex during electrically induced muscle contraction in baro-intact and baro-denervated WKY and SHR rats (Fig. 8). In WKY rats, baro-denervation enhanced the MAP and HR responses to electrically stimulated hindlimb muscle contraction as compared to the responses in baro-intact animals. Similar results were observed in baro-denervated SHR rats. In addition, the magnitude of the pressor and tachycardic responses to muscle contraction in baro-denervated WKY rats was less than that in baro-intact and baro-denervated SHR animals. In all animals, neuromuscular blockade abolished the circulatory response to electrical stimulation of the L4 and L5 ventral roots at 3, 5 and 10 times motor threshold (data not shown).
Figure 8. Effects of baro-denervation on the pressor and tachycardic responses to activation of the exercise pressor reflex in WKY and SHR animals.
As compared to their baro-intact (intact) counterparts, sino-aortic baro-denervated (SAD) WKY and SHR rats exhibited larger increases in MAP and HR in response to static muscle contraction. *P < 0.05 compared to baro-intact WKY rats. †P < 0.05 compared to baro-denervated WKY rats. §P < 0.05 compared to baro-intact SHR rats.
To ensure that the baro-denervation procedure was effective, baroreflex function was assessed during a phenylephrine-induced hypertensive challenge. In baro-intact rats, an 80 ± 10 mmHg rise in MAP caused HR to decrease by 42 ± 9 beats min−1. In baro-denervated animals, a similar increase in MAP (74 ± 11 mmHg) elicited a reduction in HR of only 6 ± 2 beats min−1.
Discussion
Using a recently developed decerebrate rat model of exercise, we have demonstrated for the first time that the abnormal cardiovascular response to physical activity in hypertension is driven, in part, by an overactive exercise pressor reflex. This conclusion is derived from the finding that the pressor and tachycardic responses to activation of the exercise pressor reflex were significantly augmented in hypertensive rats in the absence of input from both central command and the arterial baroreflex. As such, these studies provide novel insights into the neural mechanisms that regulate the cardiovascular response to exercise in hypertension.
Pressor reflex overactivity is related to exercise intensity
It is interesting that the blood pressure response to activation of the exercise pressor reflex was found to be significantly greater in hypertensive rats as compared to normotensive animals over a wide range of work intensities. For example, the pressor response to muscle contraction was positively related to the amount of tension developed in both WKY and SHR animals. However, the slope of this relationship was significantly greater in hypertensive rats. This finding suggests that the sensitivity of the exercise pressor reflex is enhanced in hypertension. In agreement with this conclusion, the pressor response to muscle contraction was 2.7-fold and 2.2-fold greater in SHR than WKY animals at medium (i.e. 34–67% of maximal tension) and high (i.e. 68–100% of maximal tension) work intensities, respectively. Even at low work intensities (i.e. 1–33% of maximal tension), the MAP response to muscle contraction in SHR rats was twice that produced in WKY rats. These results suggest that at low to moderate work intensities, the exercise pressor reflex is capable of driving abnormally large pressor responses to exercise. Theoretically, this could include relatively non-strenuous exercise activities such as lifting small to medium weight loads. These findings may prove important in determining the frequency, intensity and duration of exercise that can be safely prescribed for hypertensive individuals.
Pressor reflex overactivity and ventricular function
The exaggerated increases in MAP and HR mediated by the exercise pressor reflex in hypertensive animals occurred despite a decrease in left ventricular function. Given that MAP is determined by the product of cardiac output and systemic vascular resistance, the augmented blood pressure response to contraction in SHR rats was most probably the result of sympathetically mediated increases in systemic vascular resistance. Supporting this conclusion, sympathetic blockade with hexamethonium induced larger decreases in baseline MAP in hypertensive rats as compared to normotensive animals in this study, indicating that an enhanced basal sympathetic drive was present in the hypertensive rat model used. Further, sympathetic blockade with both hexamethonium and phentolamine completely abolished the exaggerated pressor response to muscle contraction in SHR rats, consistent with exercise pressor reflex overactivity.
Prolonged exposure to elevated systemic pressures often evokes hypertrophic remodelling of cardiac tissue that contributes to the development of ventricular dysfunction (Takimoto et al. 2005). This pathological sequence of events can eventually lead to heart failure. We have recently determined that the cardiovascular response to activation of the exercise pressor reflex is accentuated in rats with heart failure (Smith et al. 2003a, 2005a, b). Therefore, it is important to establish that the alterations in exercise pressor reflex function reported in hypertensive rats are independent of the development of heart failure. Although SHR animals displayed heart weight to body weight and heart weight to tibial length ratios consistent with the presence of cardiac hypertrophy, they did not exhibit the increases in lung weight to body weight ratios characteristic of heart failure. Further, the decreases in FS observed in SHR rats, although significant, were modest compared to those associated with enhancement of exercise pressor reflex function in heart failure (Smith et al. 2003a, 2005a, b). For example, we have previously shown that the largest augmentations in the pressor response to muscle contraction in heart failure occur in animals with reductions in FS of approximately 50% as compared to healthy rats (e.g. a mean reduction in FS from 46% in normal rats to 23% in animals with heart failure) (Smith et al. 2003a). In the current study, alterations in exercise pressor reflex function were observed in SHR rats displaying decreases in FS of less than 20% (e.g. a mean reduction in FS from 46% in WKY to 37% in SHR rats). In spite of the small decrease in FS, the magnitude of the enhanced MAP response to activation of the exercise pressor reflex in hypertensive rats was markedly greater than that previously observed in animals with heart failure (Smith et al. 2003a, 2005a, b). Lastly, heart failure has been shown to develop in SHR animals at approximately 18 months of age (Mirsky et al. 1983) whereas the animals used in the current study were 3.5–5 months old. Collectively, these findings suggest that the hypertension-induced changes in exercise pressor reflex function reported in this investigation occurred independently of the onset of heart failure.
Interactions between the exercise pressor reflex, central command and the arterial baroreflex in hypertension
Accumulating evidence suggests that the function of the exercise pressor reflex can be modulated by input from central command. For example, it has been demonstrated that electrical stimulation of the mesencephalic locomotor region (a putative component of the central command pathway) inhibits the discharge of neurons within the superficial laminae and deep portions of the dorsal horn (Degtyarenko & Kaufman, 2000b, c); neurons that receive afferent input from mechanically and chemically sensitive fibres within skeletal muscle. These findings suggest that centrally evoked motor command can inhibit the discharge of neurons receiving input from afferent fibres associated with the exercise pressor reflex (Degtyarenko & Kaufman, 2000a). Given this known interaction, it is possible that a decrease in the ability of central command to inhibit exercise pressor reflex function could contribute to the exaggerated cardiovascular response to physical activity in hypertension. However, no data currently exist evaluating the function of central command in hypertension. Therefore, it is unclear whether central command activity is increased, decreased or unchanged during exercise in hypertension. As such, it is difficult to determine the impact changes in central command activity may have on exercise pressor reflex function in hypertension. It is clear from this investiagation, however, that the exercise pressor reflex maintains the ability to drive the exaggerated cardiovascular response to exercise in hypertension independent of central command input. Future studies designed to determine the contribution of central command to the development of exercise pressor reflex overactivity in hypertension are warranted.
Likewise, the arterial baroreflex and the exercise pressor reflex are known to modify one another functionally during exercise (Papelier et al. 1997; Potts et al. 1998; McIlveen et al. 2001; Smith et al. 2003b). For example, it has been demonstrated previously that the cardiovascular response to activation of the exercise pressor reflex is enhanced in normotensive baro-denervated cats (Waldrop & Mitchell, 1985); a finding reproduced in normotensive WKY rats in this investigation. These findings suggest that the baroreflex buffers exercise pressor reflex activity under normal conditions. Impairments in arterial baroreflex function accompany the pathogenesis of hypertension such that the sensitivity (i.e. gain) of the baroreflex is diminished in hypertensive patients and animals (Lanfranchi & Somers, 2002; Minami et al. 2003). Therefore, it is possible that the exercise pressor reflex overactivity that develops in hypertension is due to a decrease in the buffering capacity of the baroreflex as has been demonstrated in other animal models of cardiovascular disease (Kim et al. 2005). If this is the primary mechanism responsible for the exaggerated cardiovascular response to muscle contraction in hypertension then it would be expected that baro-denervating normotensive animals would recapitulate the enhanced cardiovascular response to contraction seen in baro-intact SHR rats. In this study, however, the MAP and HR responses to activation of the exercise pressor reflex were greater in baro-intact SHR animals than in baro-denervated WKY rats. Furthermore, the cardiovascular response to muscle contraction was further enhanced in SHR animals by eliminating input from the baroreflex. This suggests that the baroreflex maintains the ability to buffer exercise pressor reflex function in hypertensive rats. As a result, it is concluded that although a reduction in baroreflex function may contribute to exercise pressor reflex overactivity in hypertension, it is not the sole mechanism by which exercise pressor reflex dysfunction manifests.
Relevance and potential applications
As stated, the circulatory response to exercise in hypertension is often characterized by exaggerated elevations in BP and HR (Pickering, 1987; Kahn, 1991; Kazatani et al. 1995) that may increase the risk for adverse cardiac events or stroke during physical activity (Kokkinos et al. 2002). As such, the prescription of exercise as a viable and safe therapeutic option is often limited in these individuals. Data from this study suggest that the enhanced cardiovascular response to exercise in hypertension is mediated by an overactive exercise pressor reflex. Potentially, effective treatment of this reflex dysfunction could reduce the cardiovascular risks associated with exercise in hypertension. In addition, using the rat model of exercise described in this investigation, future studies can be designed to determine the cellular, molecular and functional mechanisms of this exercise pressor reflex overactivity. By determining the pathophysiology of exercise in hypertension, novel therapeutic strategies targeted at improving cardiovascular haemodynamics may be developed that reduce the risks associated with physical activity in this disease. By reducing these risks, the benefits of exercise training may be realized to a greater degree in hypertensive individuals.
Acknowledgments
This research was supported by a grant from the American Heart Association – Texas Affiliate (BGIA no. 0565139Yto S.A.S.) and the Lawson and Rogers Lacy Research Fund in Cardiovascular Diseases (to J.H.M.). The authors thank Margaret Robledo, Martha Romero and Julius Lamar Jr for their expert technical assistance.
References
- Ansorge EJ, Augustyniak RA, Perinot ML, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H1381–H1388. doi: 10.1152/ajpheart.00985.2004. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Fictive locomotion and scratching inhibit dorsal horn neurons receiving thin fiber afferent input. Am J Physiol Regul Integr Comp Physiol. 2000a;279:R394–R403. doi: 10.1152/ajpregu.2000.279.2.R394. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Stimulation of the mesencephalic locomotor region inhibits the discharge of neurons in the superficial laminae of the dorsal horn of cats. Neurosci Lett. 2000b;296:109–112. doi: 10.1016/s0304-3940(00)01629-3. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Kaufman MP. Stimulation of the MLR inhibits the discharge of dorsal horn neurons responsive to muscular contraction. Brain Res. 2000c;880:178–182. doi: 10.1016/s0006-8993(00)02738-4. [DOI] [PubMed] [Google Scholar]
- Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33:S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- Kahn JF. The static exercise-induced arterial hypertension test. Presse Med. 1991;20:1067–1071. [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscle contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kazatani Y, Hamada M, Shigematsu Y, Hiwada K, Kokubu T. Beneficial effect of a long-term antihypertensive therapy on blood pressure response to isometric handgrip exercise in patients with essential hypertension. Am J Ther. 1995;2:165–169. doi: 10.1097/00045391-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and resting blood pressure: a meta-analytic review of randomized, controlled trials. Prev Cardiol. 2001;4:73–80. doi: 10.1111/j.1520-037x.2001.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O'Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H2416–H2423. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- Kiveloff B, Huber O. Brief maximal isometric exercise in hypertension. J Am Geriatr Soc. 1971;19:1006–1012. doi: 10.1111/j.1532-5415.1971.tb02221.x. [DOI] [PubMed] [Google Scholar]
- Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehab. 2002;22:178–183. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Krieger EM. Carotid occlusion in the rat: circulatory and respiratory effects. Acta Physiol Lat Am. 1963;13:350. [PubMed] [Google Scholar]
- Krieger EM. Neurogenic hypertension in the rat. Circ Res. 1964;15:511–520. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscle work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory response originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlveen SA, Hayes SG, Kaufman MP. Both central command and the exercise pressor reflex reset the carotid sinus baroreflex. Am J Physiol Heart Circ Physiol. 2001;280:H1454–H1463. doi: 10.1152/ajpheart.2001.280.4.H1454. [DOI] [PubMed] [Google Scholar]
- Mancia G, Mark AL. Arterial baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1983. pp. 755–793. [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Gonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- Minami N, Yoshikawa T, Kataoka H, Mori N, Nagasaka M, Kurosawa H, Kanazawa M, Kohzuki M. Effects of exercise and beta-blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. Am J Hypertens. 2003;16:966–972. doi: 10.1016/s0895-7061(03)01010-0. [DOI] [PubMed] [Google Scholar]
- Mirsky I, Pfeffer JM, Pfeffer MA, Braunwald E. The contractile state as the major determinant in the evolution of left ventricular dysfunction in the spontaneously hypertensive rat. Circ Res. 1983;53:767–778. doi: 10.1161/01.res.53.6.767. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Muntzel MS, Abe A, Petersen JS. Effects of adrenergic, cholinergic and ganglionic blockade on acute depressor responses to metformin in spontaneously hypertensive rats. J Pharmacol Exp Ther. 1997;281:618–623. [PubMed] [Google Scholar]
- Papelier Y, Escourrou P, Helloco F, Rowell LB. Muscle chemoreflex alters carotid sinus baroreflex response in humans. J Appl Physiol. 1997;82:577–583. doi: 10.1152/jappl.1997.82.2.577. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Pickering TG. Pathophysiology of exercise hypertension. Herz. 1987;12:119–124. [PubMed] [Google Scholar]
- Potts JT, Hand GA, Li J, Mitchell JH. Central interaction between carotid baroreceptors and skeletal muscle receptors inhibits sympathoexcitation. J Appl Physiol. 1998;84:1158–1165. doi: 10.1152/jappl.1998.84.4.1158. [DOI] [PubMed] [Google Scholar]
- Quintin L, Gillon JY, Saunier CF, Ghignone M. Continuous volume infusion improves circulatory stability in anesthetized rats. J Neurosci Methods. 1989;30:77–83. doi: 10.1016/0165-0270(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Radaelli A, Mircoli L, Mori I, Mancia G, Ferrari A. Nitric oxide-dependent vasodilation in young spontaneously hypertensive rats. Hypertension. 1998;32:735–739. doi: 10.1161/01.hyp.32.4.735. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mammen PPA, Mitchell JH, Garry MG. The role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003a;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005a;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- Smith SA, Querry RG, Fadel PJ, Gallagher KM, Stromstad M, Ide K, Raven PB, Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol. 2003b;551:1013–1021. doi: 10.1113/jphysiol.2003.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005b;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Mitchell JH. Effects of barodenervation on cardiovascular responses to muscle contraction. Am J Physiol. 1985;249:H710–H714. doi: 10.1152/ajpheart.1985.249.4.H710. [DOI] [PubMed] [Google Scholar]