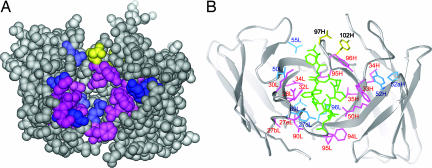
Fig. 4.
Mapping of functionally important residues on the crystal structure of preS1 peptide-bound HzKR127 Fab. (A) Space-filling representation of the interface of the Fab. The variable domain in the Fab is shown in gray. The hot spots, warm spots, and affinity-enhanced spots are colored as follows: pink (>900-fold decreased affinity relative to WT; ΔΔGD, >4 kcal/mol), blue (10- to 900-fold decreased affinity; ΔΔGD, 1.4–4 kcal/mol), and yellow (>10-fold increased affinity; ΔΔGD, less than −1.4 kcal/mol). Tyr102H (labeled in 102H) is colored yellow because the double-alanine mutation of both Asp97H and Tyr102H increased the affinity by 25-fold. (B) Ribbon representation of the HzKR127 Fab bound to preS1 peptide. The color coding is the same as in A. The bound preS1 peptide is drawn as green sticks. Among the hot spots, Gly91L is not displayed because of the absence of its side chain.