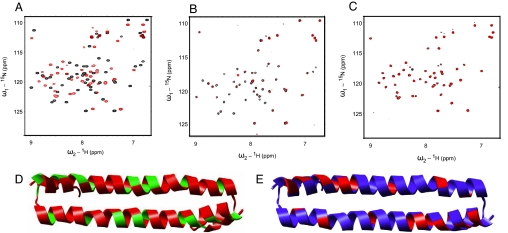
Fig. 3.
Binding of the human Mst1 SARAH domain (432–480) to the human Rassf5 SARAH domain (220–270) and the human Sav SARAH domain (residues 321–373). (A–C) Chemical shift perturbations after the addition of 0.3 mM Rassf5 SARAH domain (A), 0.6 mM Sav SARAH domain (B), and 0.1 mM Rassf5 SARAH domain and 0.6 mM Sav SARAH domain (C) to 0.3 mM (A) and 0.1 mM (B and C) 15N-labeled Mst1 SARAH dimer, respectively. The chemical shifts are monitored in 1H,15N correlation spectra. The spectra of the free protein are shown in black, and the spectra after the addition of Rassf5 or Sav SARAH domain are shown in red (A and B). The spectra of a 1:1 mixture of the Mst1 and Rassf5 SARAH domain are shown in black, and the spectra after addition of Sav SARAH domain are shown in red (C). (D and E) Ribbon representation of the human Mst1 SARAH dimer colored according to chemical shift changes or broadening effects upon addition of the human Rassf5 SARAH domain (D) and Sav SARAH domain (E) (see A and B). In D, weighted average of the 15N and 1H chemical shift perturbations Δδ = (δH2 + δN2/5)1/2 of Mst1 SARAH domain on the addition of Rassf5 SARAH domain were calculated, and the residues with Δδ > 0.12 ppm are colored red, other residues are green. In E, addition of the Sav SARAH domain to the Mst1 SARAH domain resulted in signal broadening for residues of the Mst1 SARAH domain. The residues with signal broadening, defined by peak intensities decreased to <40% of their original values, are colored red, other residues are blue. The same orientation as in Fig. 1A is shown.