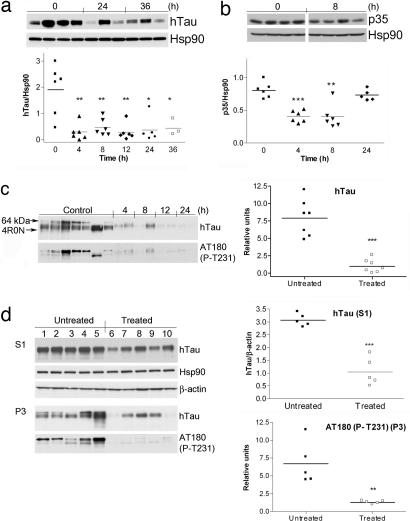
Fig. 4.
In vivo degradation of aberrant neuronal Hsp90 clients leads to a reduction in aggregated and hyperphosphorylated Tau. Western blot analysis of brain soluble fractions (S1) extracted from JNPL3 female mice treated for the indicated times with 75 mg/kg PU-DZ8. (a) Subcortical brain region of 2.5- to 4-month-old mice is presented. Human Tau levels were normalized to those of Hsp90. (b) Subcortical brain region of 2.5- to 4-month-old mice is presented. P35 levels were normalized to those of Hsp90. Representative data are displayed. (c) (Left) Western blot analysis of the insoluble Tau (P3) fractions extracted from the subcortical brain region of 6-month-old mice treated with 75 mg/kg PU-DZ8 for the indicated times. The location of 64-kDa Tau (arrowhead) and recombinant Tau containing four-repeat Tau isoforms without the N-terminal inserts (4R0N) is indicated. (Right) Relative hTau protein expression and Tau phosphorylation at T231 in the untreated versus the treated mouse group is presented. (d) Western blot analysis of soluble (S1) and insoluble (P3) subcortical brain fractions extracted from JNPL3 female mice treated for 30 days with Hsp90 inhibitors. β-Actin and Hsp90 were used as protein quantification control.