Figure 2.
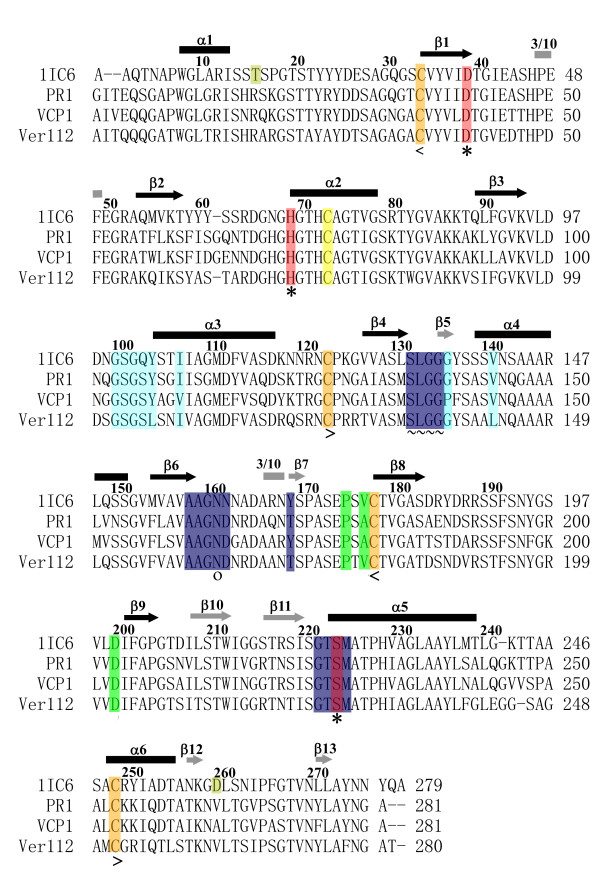
Multiple sequence alignment of the 1IC6, PR1, VCP1 and Ver112. The common secondary structure elements are marked with black arrows for parallel β strands, gray arrows for antiparallel β strands, black rods for α helices, and gray rods for 3/10 helices. Residues belonging to the S1 site are shaded blue and residues belonging to the S4 site are shaded light blue. ~ represents residues that are involved in both the S1 and S4 sites. Residues forming the catalytic triad (Asp39, His69 and Ser224, 1IC6 numbering) are shaded red and marked with an *. The oxyanion hole residue Asn161 is marked with o. Cysteines involved in the disulfide bridge are shaded orange and marked with < and > (<Cys34–Cys123> and <Cys178–Cys249>), the free Cys73 is shaded yellow. Residues forming the "strong" calcium binding site (Pro175, Val/Ala177 and Asp200) Ca1 are shaded green and residues forming the weak calcium binding site Ca2 (Thr16 and Asp260) which are unique for 1IC6 are shaded pale green.
