Figure 1.
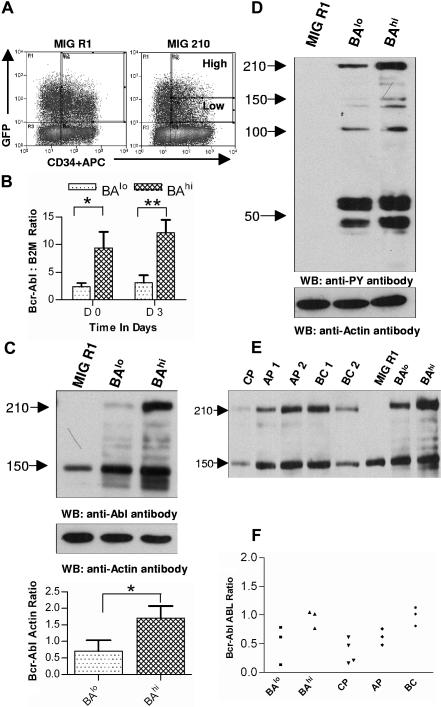
Selection of CD34+ cells expressing high and low levels of Bcr-Abl. (A) Human CD34+ cells were transduced with MIG R1 and MIG 210 retrovirus as described in “Patients, materials, and methods.” Transduced CD34+ cells were selected by flow cytometry sorting. CD34+GFP-low (BAlo) and CD34+GFP-high (BAhi) populations were selected from MIG 210–transduced cells and a single CD34+GFP+ population was selected from MIG R1–transduced cells as shown. (B) Bcr-Abl mRNA expression in transduced cells was assessed using quantitative RT-PCR and expressed as the ratio of Bcr-Abl/β2M as described in “Patients, materials, and methods.” The results shown are for freshly sorted cells (day 0) and for cells cultured for 72 hours (on day 3). Significance levels for differences between BAlo and BAhi cells were *P < .02 and **P < .036. (C) Bcr-Abl protein expression in transduced cells was assessed by Western blotting. Cells were cultured for 48 hours after sorting and protein extracts prepared as described in “Patients, materials, and methods.” Western blotting was performed using anti-Abl antibodies. Blots were reprobed with an antiactin antibody to confirm equal sample loading. Significance levels between BAlo and BAhi cells is *P < .026. (D) Protein tyrosine phosphorylation in transduced cells was assessed by Western blotting using an antiphosphotyrosine (anti-PY) antibody. (E) Protein extracts were obtained from transduced CD34+ and CD34+ cells isolated from samples obtained from CML patients, and Western blotting was performed with anti-ABL and antiactin antibodies. Results from a representative experiment are shown. (F) Bcr-Abl/ABL ratios obtained by densitometric analysis of BAlo and BAhi cells (n= 3) and primary CML CD34+ cells (n= 10) are shown.