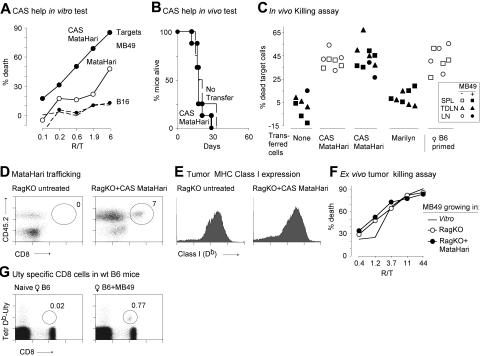
Figure 3.
Both helpless and helped MataHari CD8 cells are effective killers but cannot reject the MB49 tumor. (A) Concanavalin A supernatant (CAS), as a source of helper factors, improves killing activity of MataHari. MataHari cells were cultured without (open circles) or with (filled circles) CAS and then tested in vitro (one representative experiment out of 3) against MB49 or B16 tumor targets (solid or dashed lines, respectively) in a 4-hour P-JAM Test (“Materials and methods”). (B) In vivo activity of “helped” MataHari cells. MataHari cells were stimulated in vitro in the presence of CAS and then adoptively transferred into RAG.KO recipients that had been challenged with MB49 tumor cells one day previously. Data are from 2 pooled experiments, of which one is the same experiment as shown in A. (C) Presence of the MB49 tumor does not inhibit the in vivo killing activity of MataHari. In vivo killing activity was measured, at day 20 or 28, in tumor-bearing RAG.KO recipient mice (closed symbols) and in non—tumor-bearing controls (open symbols) that had been infused with either CAS-MataHari cells or naive Marilyn cells at day 1 after tumor challenge. To assess the male-specific killing activity, equal numbers of CFSE-labeled male and female target splenocytes were given, and the remaining target cells in SPL (squares), inguinal and axillary TDLN (triangles) and non—tumor-draining contralateral LN (circles) analyzed 18 to 24 hours later. Data are pooled from 2 independent experiments. (D and E) Analysis of MHC expression and MataHari trafficking in the tumor. MB49 tumors were collected 20 days after challenge, from untreated or CAS-MataHari treated RAG.KO (as in A) and analyzed by FACS, gating on 7-AAD− cells. (D) Presence of MataHari. Number represents the percentage of CD8 cells, found at the tumor site in one representative mouse. (E) Class I (Db) staining of tumor cells (gating on CD45.2− cells). Data in D and E are each from 2 mice representative of 10 analyzed. (F) Tumors growing in the presence of MataHari cells are not escape variants. Large tumors growing in RAG.KO mice (420 mm3), or in RAG.KO into which MataHari CD8 cells had been transferred (500 mm3), were excised when required by Animal Care and Use Committee protocols (19 days after adoptive transfer of the CD8 cells). Tumors were labeled with [3H]thymidine overnight and used as targets for previously in vitro activated MataHari cells in a 24-hour JAM Test. (G) Presence of anti-H-Y CD8 cells in normal B6 mice. Db/Uty tetramer staining in blood of a naive female B6 mouse (left panel) and of a moribund tumor-bearing female B6 mouse (right panel). Numbers represent the percentage of CD8 cells that bind to the tetramers. Two mice representative of 16.