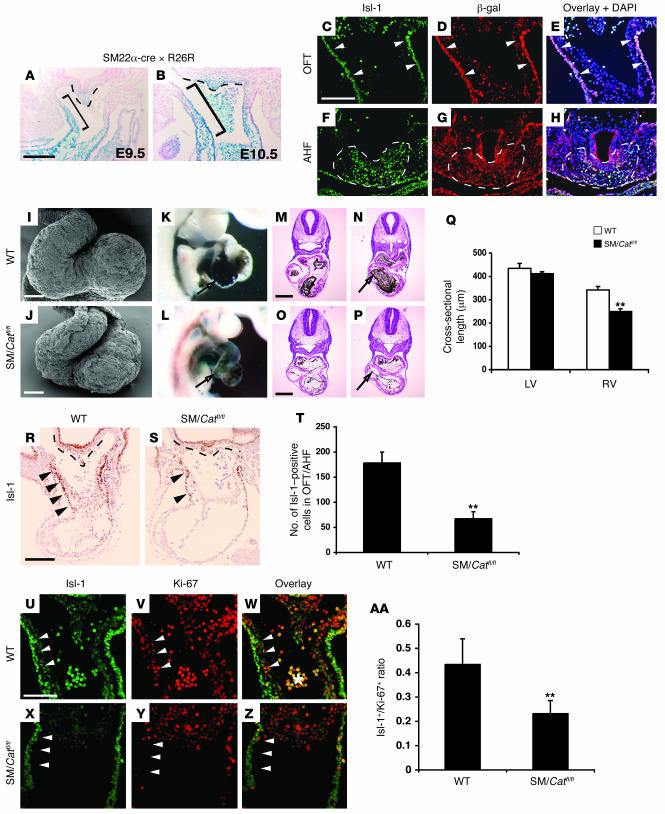
Figure 2. Loss of Wnt/β-catenin signaling in the AHF leads to decreased right heart development and loss of Isl-1 progenitors.
(A and B) SM22α-cre is active in the AHF, demonstrated by lacZ expression throughout the outflow tract (brackets) and in the pharyngeal mesodermal apex (dotted lines) of SM22α-cre × R26R mice at E9.5 and E10.5. (C–H) Immunofluorescent staining for Isl-1 and β-galactosidase expression shows extensive overlap within the outflow tract (arrowheads) and AHF (dotted lines) at E9.5. Loss of β-catenin using the SM22α-cre transgenic line caused hypoplastic right ventricle as assessed by scanning electron microscopy (I and J) and ink injections of wild-type (K) and SM22cre/Catnbflox/flox (SM/Catfl/fl) embryos (L) at E9.5. Histological sectioning at multiple levels showed the reduction in right ventricle size (arrows) at E9.5 in SM22cre/Catnbflox/flox (O and P) compared with wild-type embryos (M and N). (Q) Right ventricular diameter in SM22cre/Catnbflox/flox compared with wild-type embryos. (R–T) To assess changes in Isl-1 AHF progenitors, wild-type and SM22cre/Catnbflox/flox embryos were immunostained for Isl-1 protein expression. SM22cre/Catnbflox/flox mutants have severely reduced numbers of Isl-1 AHF progenitors in the outflow tract at E9.5. (U–AA) Isl-1 and Ki-67 double immunofluorescence was performed to determine changes in proliferation in AHF progenitors. Reduced Ki-67 staining in Isl-1–positive cells within the outflow tract was observed in SM22cre/Catnbflox/flox mutant embryos (arrowheads). (AA) Quantitation showed an almost 50% reduction in Isl-1 AHF progenitor proliferation. **P < 0.005. Scale bars: 100 μm (A–J); 500 μm (M–P); 125 μm (R and S); 75 μm (U–Z).