Fig. 6.
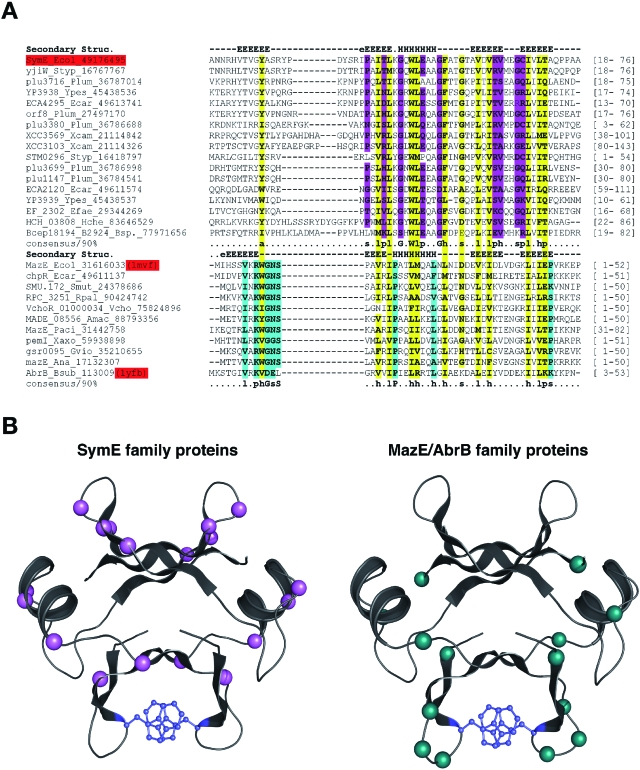
SymE is an AbrB superfamily member that has acquired a toxin-like function. A. Multiple alignment of the SymE family and other members of the AbrB superfamily. Proteins are denoted by gene name, species abbreviation, and GenBank Identifier (gi) number; separated by underscores. Positions strongly conserved at or above the 90% applied on the entire family of proteins only among the classic SymE family proteins are shaded pink, whereas those similarly conserved only in the classic MazE/AbrB family are shaded aqua and those conserved between both are shaded yellow. Consensus similarity designations are as follows: h, hydrophobic residues (ACFILMVWY); s, small residues (AGSVCDN); p, polar residues. Secondary structure assignments obtained from the jpred prediction for the SymE family and from the crystal structures (like PDB: 1mvf and 1ub4) for the rest of the AbrB superfamily are shown above the alignment where E represents a strand and H represents a helix. The region shown in the alignment spans the entire length of the DNA binding domain of the classical AbrB proteins. The boundaries are shown to the right. Species abbreviations are as given for the full alignment in Fig. S2. B. Models of the classic SymE family and classic MazE/AbrB family proteins. An idealized version of the AbrB fold was constructed using the consensus sequence derived from the hidden Markov model for the entire fold using SWISSMODEL server of SWISSPDB and the 1mvf structure as a template; in both cases the structure is depicted as a dimer formed by two interlocking monomers, which was achieved using the oligomer mode in SWISSMODEL (Guex and Peitsch, 1997). The positions that are conserved in the SymE family at the 90% consensus are coloured pink as in Fig. 6A. The majority of them line a groove on one face of the protein. This surface faces away from the surface that contains the most conserved positions unique to the classical members of the MazE/AbrB superfamily (coloured aqua). The uniquely conserved regions include polar residues that could potentially mediate the key interactions of SymE with the ribosome or target RNA. The aromatic residues involved in stabilizing the dimer through a π–π stacking interaction are shown in blue.