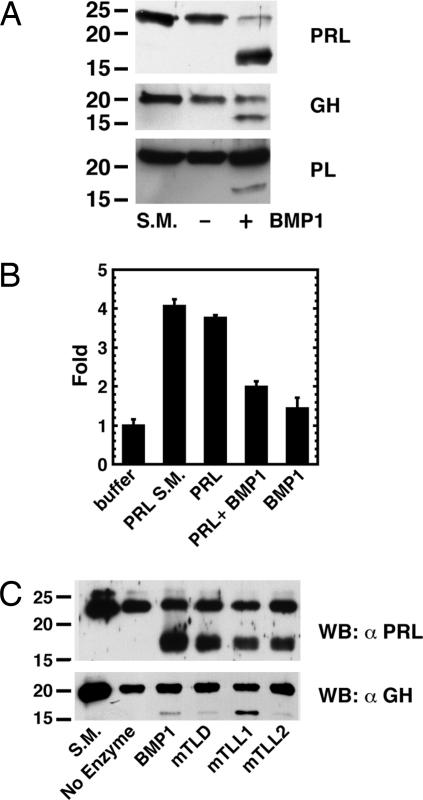
Fig. 2.
BMP1-like proteinases cleave PRL, GH, and PL to ≈17-kDa fragments. (A) Hormones were incubated for 6 h in the presence (+) or absence (−) of wild-type BMP1 and then subjected to SDS/PAGE and Western blotting with antibodies to the relevant hormone. S.M., starting material before incubation. (B) 293 cells cotransfected with a PRL receptor construct and a reporter gene containing three copies of the consensus Stat5 binding sequence upstream of the luciferase gene were incubated with buffer, PRL starting material (S.M.), BMP1 alone, or PRL incubated for 24 h in the absence or presence of BMP1. Levels of luciferase activity were normalized to levels of β-galactosidase activity from a cotransfected β-galactosidase expression construct to control for transfection efficiency. Levels of luciferase activity are in arbitrary units normalized to a value of 1 for buffer alone. Data are given as means ± SD (n = 3). A Student t test, performed with SigmaPlot, showed a significant difference between levels of luciferase activity for the PRL and PRL plus BMP1 samples (P < 0.003). (C) PRL and GH were incubated for 6 h alone (No Enzyme) or in the presence of BMP1, mTLD, mTLL1, or mTLL2 and then subjected to SDS/PAGE and Western blot analysis with antibodies to the relevant hormone.