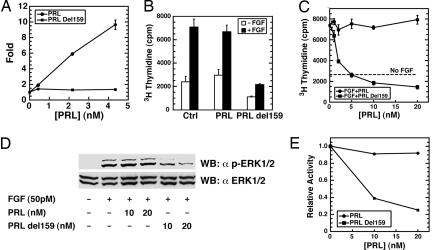
Fig. 4.
Truncated recombinant PRL representing the ≈17-kDa N-terminal BMP1 cleavage product inhibits endothelial cell proliferation and ERK1/2 phosphorylation. (A) In the same type of reporter assay used in Fig. 2B, PRLdel159 is shown to lack the Jak/Stat signaling activity shown by full-length PRL. (B) HUVECs in the presence or absence of 50 pM bFGF were treated with 20 nM full-length PRL or PRL del159 followed by determination of [3H]thymidine incorporation. A Student t test, performed with SigmaPlot, showed PRLdel159 (P < 0.003), but not full-length PRL (P < 0.24), to significantly differ from buffer alone in the ability to inhibit FGF-induced [3H]thymidine incorporation by HUVECs. (C) HUVECs in the presence or absence of 50 pM bFGF were treated with various amounts of full-length and del159 PRL, and thymidine incorporation was determined as in B. Data for A–C are given as means ± SD (n = 3). (D) Extracts of HUVECs treated with PRL or PRLdel159 in the presence (+) or absence (−) of FGF were subjected to SDS/PAGE and then Western blotting with antibody specific for phosphorylated ERK1/2 (α p-ERK1/2) or total ERK1/2 (α ERK1/2). (E) The data of D were quantified and plotted by using scanning densitometry and NIH Image software.