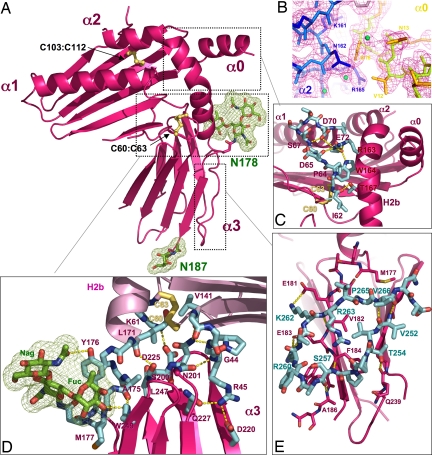
Fig. 1.
The 3D structure of m157. (A) Ribbon diagram of m157 showing an overall MHC class I-like structural architecture. Disulphide bonds are shown as yellow sticks, and two glycosylation sites are shown in green. (B) The experimental electron density map contoured at 1.5 ó, showing the interactions between the N-terminal α0 helix and the H2b segment of the α2 helix. (C) A network of side- and main-chain (shown in cyan) hydrogen-bonding interactions exists between the α1 and α2 domains. (D) The hinge region between the platform has extensive contacts between the α3 domain loops and the underside of the α1/α2 platform. Additional contacts are contributed by the N-linked glycosylation located at Asn-178 (shown in stick and mesh representation; green). (E) A unique C-terminal extension forms a circular loop that mediates contacts between the two β-sheets of the α3 domain's Ig sandwich (cyan-colored sticks). Hydrogen-bonding interactions are shown as dotted yellow lines, and disulfide bonds are shown in ball and stick representation (yellow).