Abstract
Isoniazid (INH) resistance is most frequent among drug-resistant Mycobacterium tuberculosis clinical isolates. This study was conducted to investigate whether INH could induce its own resistance. During INH susceptibility testing in BACTEC 12B and MGIT 960 media, weekly subcultures were made from the drug-containing media into fresh medium without drug and susceptibility testing was performed. Rifampin (RIF) was used as a control drug. M. tuberculosis H37Rv and three clinical isolates were tested in this study. INH-resistant subcultures were analyzed for catalase activity, INH susceptibility, and mutations associated with INH resistance. With inoculum size (104 bacilli) smaller than a size that contains spontaneously INH-resistant mutants, INH was found to induce resistance to itself in INH-tolerant persisters but not to other drugs. The minimum time required for induction of INH resistance was 5 to 6 days. In contrast, RIF did not induce RIF resistance. Eight subcultures with INH-induced resistance were analyzed, and two had a MIC of 0.4 μg/ml INH and six had MICs of over 2 μg/ml INH. Four of the eight subcultures with INH-induced resistance had lost catalase activity, with three having katG mutations. Despite being a powerful frontline tuberculosis drug, INH has the potential drawback of inducing its own stable genetic resistance in INH-tolerant persisters. This finding helps to explain the higher frequency and prevalence of INH-resistant isolates than isolates with resistance to other drugs in patients.
Isoniazid (INH) is an important first-line tuberculosis (TB) drug that, along with rifampin (RIF) and pyrazinamide, forms the basis of the widely used directly observed treatment short course for the treatment of TB (16). INH is highly active against Mycobacterium tuberculosis, with MICs of 0.01 to 0.25 μg/ml, but the activity of INH is demonstrated only for growing tubercle bacilli, not nongrowing bacilli (21). INH is a prodrug that requires activation by the M. tuberculosis catalase-peroxidase (KatG) (18) to its active species including isonicotinic acyl radicals (10, 11) and reactive oxygen species (12). The molecular target of INH is InhA (1), an NADH-dependent enoyl acyl carrier protein reductase, involved in cell wall mycolic acid synthesis.
Shortly after INH was introduced in clinical treatment of TB in 1952, M. tuberculosis strains resistant to INH, many of which had defective catalase activity, were reported (8). INH resistance is primarily mediated by mutations in katG (18) and inhA (1). Mutation in the katG gene which leads to loss of or reduced catalase-peroxidase activity is a major mechanism of INH resistance in M. tuberculosis (20, 22). Mutations in the inhA encoding the drug target or mutations in its promoter can cause INH resistance (1). Mutations in ndh, encoding type II NADH dehydrogenase, could also potentially be as involved in INH resistance (7, 9, 14).
Resistance to INH is the most frequent among all drug-resistant clinical isolates, with incidence as high as 20 to 30% in some regions (4). Strains resistant to INH and RIF (multidrug resistant) or other drugs are a significant public health concern and threaten the effective control of TB (6; World Health Organization Global Tuberculosis Program [http://www.who.int/gtb/]). It is unclear whether the high incidence of INH resistance is due to frequent use of the drug as monotherapy or to the intrinsic property of INH of inducing its own resistance. Resistance to INH develops readily, with a high frequency of 10−6 (17), which is higher than that of most TB drugs, such as RIF, which has a mutation frequency of 10−7 to 10−8 (17). The basis for this high mutation frequency to INH resistance is unclear but may be related to the reactive oxygen radicals generated during INH activation, which can cause mutagenesis of DNA. While the mutation frequency determination is done with actively replicating cultures, it is unclear whether the nonreplicating bacteria that are not killed by INH, i.e., INH persisters (which are nonreplicating and show phenotypic resistance but not genetic resistance and are still susceptible to antibiotics upon subculture), can develop mutations during exposure to INH. This study was conducted to evaluate this possibility.
MATERIALS AND METHODS
Study protocol.
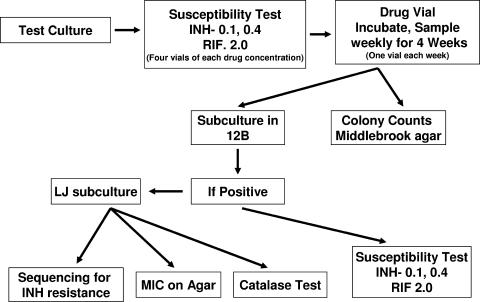
An outline of the protocol is given in Fig. 1. M. tuberculosis H37Rv and three clinical isolates TB 7, TB 19, and TB 20, which were obtained from U.S. TB patients, were used in this study. All these strains were susceptible to 0.1 μg/ml INH and also to 1 μg/ml RIF as tested by the BACTEC or MGIT method. The inoculum for antibiotic susceptibility testing (AST) was prepared and CFU were established for each inoculum. The target inoculum for 12B medium was 104 to 105 CFU/ml, and that for MGIT medium was 106 to 107 CFU/ml. BACTEC 460 susceptibility testing was carried out according to the standard procedure (13). MGIT susceptibility testing was carried out as recommended by the manufacturer. One growth control medium and four drug-containing media for each concentration were set up. Once the growth index (GI) of the control vial reached 30 or more, results were interpreted. The drug-containing vials were further incubated and tested on BACTEC 460 instruments three times a week for a total of 4 weeks. Also, weekly CFU counts from drug-containing vials were made by plating out dilutions on Middlebrook 7H11 agar. On weeks 1, 2, 3, and 4, 0.1 ml of the culture in one drug-containing vial from each set of INH concentrations was subcultured into vials of fresh 12B medium without drug. The subculture vials were incubated and tested two or three times per week until the GI reached 10 or more and then daily until the peak GI was achieved. Once the subculture from a drug-containing 12B or MGIT medium was positive, AST was set up for INH at 0.1 and 0.4 μg/ml and for RIF at 1.0 (BACTEC 460) and 2.0 μg/ml (MGIT 960). If a subculture was resistant to INH, a subculture was made on Lowenstein-Jensen (LJ) slants. These LJ subcultures were used for catalase and molecular testing. Throughout the study the incubation temperature was 37°C.
FIG. 1.
Study design.
Determination of MIC of INH-resistant cultures and catalase activity.
The INH MIC for INH-resistant cultures was determined by the agar dilution method as described previously (3). Cultures which had developed INH resistance after drug exposure were tested for susceptibility to streptomycin, RIF, and ethambutol in 12B medium to determine possible development of any secondary resistance. Catalase activity was assayed as described previously (19).
Genomic DNA isolation, PCR amplification, and sequencing analysis of katG, inhA, and ndh genes.
Genomic DNA was isolated as previously described (19). Primers were designed from the M. tuberculosis H37Rv genome sequence (20) to amplify the whole 2.2-kb katG, the 1.5-kb region of mabA-inhA, and the 1.4-kb ndh by PCR. The standard PCR mixture (50 μl) contained 1.5 units of HotStart Taq DNA polymerase, 1× PCR buffer supplemented with 2.5 mM MgCl2 (QIAGEN, Chatsworth, CA), 500 nM of each forward and reverse primer, 200 μM of deoxynucleoside triphosphates, and 0.1 μg of DNA template. PCR was performed with the following parameters: 95°C for 10 min, followed by 40 cycles at 94°C for 40 s, 55°C for 40 s, and a 72°C extension for 120 s and the final extension at 72°C for 10 min. The entire katG, inhA-mabA, and ndh genes contained in the PCR products were sequenced using primers that amplified these genes and also appropriate internal sequencing primers.
RESULTS
The phenomenon of late bacterial growth during INH susceptibility testing.
For INH susceptibility testing, an inoculum of 2 × 104 bacilli for H37Rv and an inoculum in the range of 2.4 × 104 to 2.2 × 105 bacilli for clinical isolates were used in BACTEC 12B medium containing 0.1 and 0.4 μg/ml INH. The GI values in the BACTEC vials were initially low due to INH inhibition but subsequently started to rise upon continued incubation for 4 weeks (Table 1). The GI values correlated with the CFU data (Table 1). Overall, the total CFU counts decreased within 1 week when mycobacteria were incubated in the INH-containing media. The decrease in CFU with the high concentration of INH (0.4 μg/ml) was greater than that with the low concentration (0.1 μg/ml) (Table 1). However, after the first week the CFU counts started rising, sometimes reaching the starting CFU level at the end of 4 weeks. In order to rule out the possibility that the bacterial growth after INH exposure for 4 weeks is due to degradation of INH in the medium, we tested the INH-containing medium after incubation on a weekly basis and found no loss of drug activity. On the other hand, in the presence of RIF (2 μg/ml), the GI values did not increase within the 4-week incubation, the CFU counts dropped considerably, and no viable bacteria were recovered even after 1 to 2 weeks of incubation (Table 1). Similar findings with INH and RIF as those found in the BACTEC 12B medium were also seen in MGIT medium (data not shown). This kind of study is possible only in liquid media, as solid media do not support growth of mycobacteria stressed by a drug as shown here.
TABLE 1.
GIs and CFU counts of M. tuberculosis H37Rv in INH and RIF susceptibility testing by the BACTEC 460 methoda
| Drug, concn (μg/ml), and culture | GI at day:
|
Final count (CFU/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8b | 15b | 22b | 28b | ||
| INH, 0.1 | ||||||||||||
| Control | 3 | 0 | 1 | 3 | 7 | 15 | 22 | 37 | ||||
| Wk 1 | 16 | 12 | 8 | 6 | 4 | 6 | 5 | 5 | 1.2 × 103 | |||
| Wk 2 | 20 | 14 | 10 | 8 | 7 | 9 | 5 | 5 | 18 | 3.1 × 103 | ||
| Wk 3 | 18 | 13 | 8 | 7 | 4 | 7 | 4 | 4 | 19 | 72 | 2.4 × 104 | |
| Wk 4 | 16 | 11 | 7 | 8 | 3 | 5 | 3 | 3 | 17 | 60 | 251 | 1.22 × 105 |
| INH, 0.4 | ||||||||||||
| Control | 3 | 0 | 1 | 3 | 7 | 15 | 22 | 37 | ||||
| Wk 1 | 15 | 9 | 0 | 6 | 4 | 5 | 4 | 2 | 55 | |||
| Wk 2 | 18 | 12 | 9 | 8 | 6 | 7 | 6 | 5 | 17 | 3.6 × 103 | ||
| Wk 3 | 17 | 10 | 7 | 6 | 4 | 6 | 5 | 4 | 14 | 93 | 1.45 × 104 | |
| Wk 4 | 15 | 11 | 8 | 7 | 4 | 0 | 4 | 3 | 9 | 36 | 106 | 1.32 × 104 |
| RIF, 2 | ||||||||||||
| Control | 3 | 0 | 1 | 3 | 7 | 15 | 22 | 37 | ||||
| Wk 1 | 10 | 5 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | |||
| Wk 2 | 11 | 8 | 3 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | ||
| Wk 3 | 10 | 6 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Wk 4 | 15 | 4 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 |
M. tuberculosis H37Rv was inoculated into vials containing 0.1 or 0.4 μg/ml INH or 2 μg/ml RIF and incubated at 37°C for 4 weeks. GI values were measured daily within the first week and then weekly for 4 weeks. Colony counts were measured once a week by taking aliquots from each vial and plating on solid media without antibiotic. The beginning CFU for this experiment is 2.0 × 104.
Time when a culture vial was taken out and subcultures and colony counts were made.
When cultures in drug-containing vials at both INH concentrations were subcultured in fresh 12B or MGIT medium at different intervals, growth was obtained with all the test cultures. Time to positive subcultures was much shorter in MGIT medium than in 12B medium. In the case of RIF, subcultures in 12B became negative after exposure of only 1 week with strains TB 19 and 20 and 3 weeks with H37Rv, while TB 7 remained positive all 4 weeks. Most of the time the subculture from 12B medium containing RIF did not show good growth, and it had to be subcultured one or two times to achieve good growth. No CFU were detected during subculture on 7H11 medium even after 1 week of exposure to RIF.
Emergence of INH resistance among the drug-exposed bacteria.
All the subcultures after the drug exposure were subjected to a second round of susceptibility testing. Subcultures made weekly from 12B vials containing 0.1 and 0.4 μg/ml INH and inoculated with H37Rv, TB 7, or TB 20 showed complete resistance to INH at 0.1 and 0.4 μg/ml while TB 19 showed susceptibility at week 1 at only the 0.4-μg/ml level and resistance for the rest of the 3 weeks (Tables 2 and 3). Cultures recovered after RIF exposure and tested for RIF susceptibility were found completely susceptible to RIF (Table 3). Cultures in MGIT medium, containing RIF, when subcultured in fresh MGIT medium grew, but very slowly (not shown). AST was performed for RIF, and all these subcultures were found susceptible to RIF (Table 3), indicating that the RIF persisters, unlike INH persisters, did not develop any RIF resistance.
TABLE 2.
Colony counts of M. tuberculosis clinical isolates after exposure to INH in 12B mediuma
| Isolate and INH concn (μg/ml) | Inoculum (CFU/vial)b | Colony count (CFU) at wk:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| TB 7 | |||||
| 0.1 | 2.4 × 104 | 1.7 × 105 | 7.6 × 103 | 2.5 × 104 | 7.9 × 104 |
| 0.4 | 2.4 × 104 | 0 | 5.6 × 102 | 1.2 × 104 | 1.1 × 105 |
| TB 19 | |||||
| 0.1 | 1.4 × 105 | 25 | 1.1 × 103 | 4.8 × 103 | 7.0 × 103 |
| 0.4 | 1.4 × 105 | 0 | 2.0 × 102 | 3.4 × 104 | 4.8 × 104 |
| TB 20 | |||||
| 0.1 | 2.2 × 105 | 2.4 × 102 | 1.4 × 104 | 1.6 × 104 | 2.5 × 104 |
| 0.4 | 2.2 × 105 | 40 | 1.3 × 104 | 7.5 × 104 | 8.0 × 104 |
Three clinical strains of M. tuberculosis were inoculated into vials containing 0.1 or 0.4 μg/ml INH and incubated at 37°C for 4 weeks. Colony counts were measured once a week by taking aliquots from each vial and plating on solid media without antibiotic.
Total CFU inoculated into each drug vial containing 4 ml of 12B medium. The counts/ml of medium will be one-fourth of this.
TABLE 3.
Drug susceptibility results after exposure to the test drugs in 12B mediuma
| Culture | Drug, concn (μg/ml) | Susceptibility resultb for subculture at wkc:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| H37Rv | INH, 0.1 | R | R | R | R |
| INH, 0.4 | R | R | R | R | |
| RIF, 2.0 | S | S | S | NG | |
| TB 7 | INH, 0.1 | R | R | R | R |
| INH, 0.4 | R | R | R | R | |
| RIF, 2.0 | S | S | S | S | |
| TB 19 | INH, 0.1 | R | R | R | R |
| INH, 0.4 | S | R | R | R | |
| RIF, 2.0 | S | NG | NG | NG | |
| TB 20 | INH, 0.1 | R | R | R | R |
| INH, 0.4 | R | R | R | R | |
| RIF, 2.0 | S | NG | NG | NG | |
The weekly subcultures of H37Rv and clinical isolates TB 7, TB 19, and TB 20 after drug exposure in 12B vials up to 4 weeks were subjected to a second round of susceptibility testing in 12B vials containing 0.1 or 0.4 μg/ml INH or 2 μg/ml RIF.
R, resistant; S, susceptible; NG, no growth.
Exposure time after which a subculture was done and susceptibility test was performed using the subculture growth.
Minimum time required for induction of INH resistance.
In order to determine the minimum time required for emergence of INH resistance, we performed daily subculture of H37Rv from cultures exposed to INH at 0.1 and 0.4 μg/ml and tested their susceptibility to INH and also determined daily CFU counts (Table 4). H37Rv culture exposed up to 4 days in 12B medium showed complete susceptibility at 0.1- and 0.4-μg/ml INH concentrations (Table 4). However, the culture developed resistance on day 5 at 0.1 μg/ml INH and on day 6 at 0.4 μg/ml INH. Once the culture developed resistance, it was complete at both low and high INH concentrations. The CFU counts in the original drug vials declined significantly up to 6 days and then started rising. Similar results were observed with MGIT medium though the overall CFU counts were higher than those for 12B medium.
TABLE 4.
Minimum time required for development of INH resistance for a subculture (H37Rv) exposed to INH in 12B mediuma
| Exposure time (day) | 0.1 μg/ml INH
|
0.4 μg/ml INH
|
||||
|---|---|---|---|---|---|---|
| CFU/ml in 12B medium | AST susceptibility result after subculture in INH at:
|
CFU/ml in 12B medium | AST susceptibility result of subculture in INH at:
|
|||
| 0.1 μg/ml | 0.4 μg/ml | 0.1 μg/ml | 0.4 μg/ml | |||
| 0 | 30,000 | S | S | 30,000 | S | S |
| 1 | 6,275 | S | S | 2,050 | S | S |
| 2 | 1,130 | S | S | 520 | S | S |
| 3 | 125 | S | S | 45 | S | S |
| 4 | 55 | S | S | 20 | S | S |
| 5 | 55 | R | R | 10 | S | S |
| 6 | 30 | R | R | 10 | R | R |
| 7 | 475 | R | R | 445 | R | R |
To determine the minimum time required for emergence of INH resistance, daily subcultures of H37Rv from INH-exposed cultures at 0.1 and 0.4 μg/ml were tested for susceptibility to INH at 0.1 and 0.4 μg/ml and CFU.
Stability of test drugs in the medium.
In order to rule out the possibility that the bacterial growth after INH exposure for 4 weeks was due to degradation of INH in the medium, we tested the INH-containing medium after incubation on a weekly basis by the BACTEC method. Drug susceptibility test results showed no loss of drug activity when the drug-containing medium was incubated at 37°C for 0, 1, 2, 3 and 4 weeks. This result suggests that INH is stable during the 4-week incubation of drug susceptibility testing.
Stability and specificity of resistance developed by INH exposure.
H37Rv exposed to 0.4 μg/ml INH that had developed resistance was subcultured, and AST from the subculture was performed for 0.1 and 0.4 μg/ml INH. The induced INH resistance was found to be stable when cultures were repeatedly subcultured without INH at least five times, testing each time for INH susceptibility. Cultures which had developed INH resistance after exposure were tested for susceptibility to streptomycin, RIF, and ethambutol and were found completely susceptible to all the three drugs.
Levels of INH resistance, catalase activity, and INH resistance mutations in INH-induced cultures.
To further characterize the INH-resistant cultures that developed after INH exposure, we isolated single clones from the exposed culture of H37Rv, four clones, SC1 to -4, from the culture exposed to 0.1 μg/ml INH, and four clones from the culture exposed to 0.4 μg/ml INH. We then determined the level of INH resistance, catalase activity, and potential mutations in INH resistance genes, katG, inhA, and ndh (Table 5). Isolates SC1 and SC5 had a MIC of 0.4 μg/ml INH, whereas the remaining six clones were all highly resistant to at least 2 μg/ml INH. The two isolates resistant at the 0.4-μg/ml level, SC1 and SC5, were still catalase positive, with slightly reduced activity compared with drug-susceptible control strain H37Rv. SC2 and SC3 had significantly reduced catalase activity. In contrast, SC4, SC6, SC7, and SC8 had no detectable catalase activity. DNA sequencing analysis revealed that isolates SC3, -6, and -7 had mutations in katG. Mutation of TGG to CGG at codon 328, causing Trp328Arg, was observed in INH-resistant clones SC6 and SC7. Mutation of GAC to CAC at codon 329, causing Asp329His, was found in INH-resistant clone SC3. No mutations in the inhA gene or its promoter or the ndh gene were identified in any strains. The other five INH-resistant isolates had no mutation in any of the known INH resistance genes, katG, inhA, and ndh. SC4 and SC8, which were catalase negative, could be due to mutations in katG regulatory genes.
TABLE 5.
Characterization of INH-resistant subcultures derived from INH-exposed culture of H37Rva
| Subculture | AST result by BACTEC | MIC (μg/ml) | Catalase activityb | Mutation in katG product |
|---|---|---|---|---|
| H37Rv control | +++ | None (WTd) | ||
| SC1 | Rc | 0.4 | ++ | None (WT) |
| SC2 | R | >2 | + | None (WT) |
| SC3 | R | >2 | + | D329H |
| SC4 | R | >2 | − | None (WT) |
| SC5 | R | 0.4 | ++ | None (WT) |
| SC6 | R | >2 | − | W328R |
| SC7 | R | >2 | − | W328R |
| SC8 | R | >2 | − | None (WT) |
INH-resistant subcultures derived from an INH-exposed H37Rv culture were characterized for level of resistance, catalase activity, and resistance mutations. SC1 to -4 and SC5 to -8 are subcultures derived from INH susceptibility testing vials inoculated with H37Rv containing 0.1 μg/ml and 0.4 μg/ml INH, respectively.
The number of + signs indicates relative catalase activity; − indicates no catalase activity.
R, resistance.
WT, wild type.
DISCUSSION
In this study we found that during INH susceptibility testing a subpopulation of tubercle bacilli that are not killed by INH (INH persisters) could develop INH resistance within 1 week of incubation in liquid media (Tables 1 and 4). In contrast, RIF did not induce RIF resistance, and the subcultures from RIF-exposed liquid media were still susceptible to RIF. These observations suggest that INH can induce INH resistance in INH persisters whereas the RIF persisters, if any, do not develop any RIF resistance. The INH-induced resistance is specific to INH, as the INH-resistant cultures were still susceptible to other TB drugs.
INH is activated by catalase-peroxidase (KatG) to produce reactive species that can potentially damage DNA and have a mutagenic property (12, 21), which could be responsible for the generation of INH-resistant mutants at a high frequency (10−6) (17). Mutation frequency is generally determined by selection of preexisting mutants present in a growing bacterial population. However, in the case of INH, the drug INH not only selects preexisting mutants in a large bacterial population but could also induce true genetic resistance in relatively small numbers of INH-tolerant persisters, as shown in this study (Tables 1 and 3). It is of interest that the minimum time required for INH resistance induction was 5 to 6 days after the exposure to INH (Table 3). Since the inoculum size of 3 × 104 bacilli used in the INH susceptibility testing was smaller than the 106 bacilli required to contain spontaneously INH-resistant mutants and since INH exposure should not allow the inoculated bacilli to grow during the 5-day incubation with INH, the emergence of INH resistance after 5 to 6 days must be due to an induced INH resistance in nongrowing INH-tolerant persister bacilli, rather than the selection of preexisting spontaneously INH-resistant mutants in a growing bacterial population. It is interesting that, on daily subculturing from INH-containing media, the colony counts started increasing sharply after day 6. Thus, INH has the capacity to induce INH resistance in addition to selection of preexisting mutants, which could be responsible for the unusually high frequency of INH-resistant mutants and the consequently high prevalence of INH resistance in the patient population.
By contrast, no viable bacteria were found when RIF-containing media were plated out on solid medium. However, subculturing from the same RIF-containing medium into fresh liquid medium did sometimes yield growth which was very slow, and cultures had to be subcultured several times to achieve good growth. Subsequent testing showed that subcultures from RIF-containing media were completely susceptible to RIF while subcultures from INH-containing media were completely resistant to INH. These findings indicate that solid medium does not show the real viability of the culture. Time to positivity in 12B medium of a drug-exposed culture was significantly longer in 12B medium than in MGIT medium. The above data suggest that bacilli exposed to INH are easier to rejuvenate than those exposed to RIF and that MGIT medium is better for the growth of drug-exposed bacilli than 12B medium while solid medium does not support the growth of the drug-exposed bacteria very well.
In a previous study, INH has been shown to induce a transient phenotypic INH resistance mediated by a reserpine-repressible efflux mechanism that is lost upon repeated subcultures in drug-free medium (15). Because no mutations were found in katG, the authors concluded that INH-induced resistance is phenotypic and caused by efflux pumps (15). However, in that study the whole katG gene was not sequenced and only the KatG315 mutation was analyzed (15). Thus, potential mutations in other parts of the katG gene could not be ruled out. In contrast, we found mutations in katG in three of eight INH-resistant isolates, indicating that INH could induce genetic resistance. Another difference between the two studies is that the whole culture or a mixed population was used for KatG315 mutation analysis, whereas this study used single clones from a subculture of INH-containing medium, which were subsequently found resistant to both 0.1 and 0.4 μg/ml INH. It is known that an INH-resistant M. tuberculosis subpopulation is at a disadvantage in competition with a drug-susceptible population (2), presumably due to deficient catalase in the INH-resistant subpopulation. Thus, upon prolonged culture of a mixed population in the absence of INH selection, the INH-resistant subpopulations in mixed cultures may be lost and the whole culture could revert to INH sensitivity over time. This is especially true for cultures that have low level of INH resistance. Our repeated subculturing indicated that the resistance was stable at least after five subcultures. In conclusion, we propose that INH in addition to selecting preexisting mutants in a large bacterial population, could induce its own resistance in small number of nonreplicating persisters that are tolerant of INH as a possible explanation for its high incidence of resistance in a clinical setting.
Acknowledgments
Y.Z. was supported by NIH grants AI44063 and AI49485 and Basic Research (973) Program (2005CB523102), China.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, M., S. Bushby, and D. Mitchison. 1953. Isoniazid-resistant strains of tubercle bacilli. Their development and stability. Lancet i:314-320. [DOI] [PubMed] [Google Scholar]
- 3.Canetti, G., W. Fox, A. Khomenko, H. T. Mahler, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smelev. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 41:21-43. [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn, D. L., F. Bustreo, and M. C. Raviglione. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. International Union Against Tuberculosis and Lung Disease. Clin. Infect. Dis. 24(Suppl. 1):S121-S130. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Espinal, M. A. 2003. The global situation of MDR-TB. Tuberculosis 83:44-51. [DOI] [PubMed] [Google Scholar]
- 7.Lee, A., A. Teo, and S. Wong. 2001. Novel mutations in ndh in isoniazid-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 45:2157-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middlebrook, G. 1954. Isoniazid resistance and catalase activity of tubercle bacilli. Am. Rev. Tuberc. 69:471-472. [DOI] [PubMed] [Google Scholar]
- 9.Miesel, L., T. R. Weisbrod, J. A. Marcinkeviciene, R. Bittman, and W. R. Jacobs, Jr. 1998. NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J. Bacteriol. 180:2459-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawat, R., A. Whitty, and P. J. Tonge. 2003. The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. Proc. Natl. Acad. Sci. USA 100:13881-13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozwarski, D. A., G. A. Grant, D. H. Barton, W. R. Jacobs, Jr., and J. C. Sacchettini. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98-102. [DOI] [PubMed] [Google Scholar]
- 12.Shoeb, H. A., B. U. Bowman, Jr., A. C. Ottolenghi, and A. J. Merola. 1985. Evidence for the generation of active oxygen by isoniazid treatment of extracts of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 27:404-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi, S. H. 1992. Antimicrobial susceptibility testing: radiometric (BACTEC) tests for slowly growing mycobacteria, p. 14-25. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. ASM Press, Washington, DC.
- 14.Vilcheze, C., T. R. Weisbrod, B. Chen, L. Kremer, M. H. Hazbon, F. Wang, D. Alland, J. C. Sacchettini, and W. R. Jacobs, Jr. 2005. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob. Agents Chemother. 49:708-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viveiros, M., I. Portugal, R. Bettencourt, T. C. Victor, A. M. Jordaan, C. Leandro, D. Ordway, and L. Amaral. 2002. Isoniazid-induced transient high-level resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2804-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2003. Treatment of tuberculosis: guidelines for national programmes, 3rd ed. World Health Organization, Geneva, Switzerland.
- 17.Winder, F. 1982. Mode of action of the antimycobacterial agents and associated aspects of the molecular biology of mycobacteria. In C. Ratledge and J. Stanford (ed.), The biology of mycobacteria. Academic Press, New York, NY.
- 18.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, Y., T. Garbe, and D. Young. 1993. Transformation with katG restores isoniazid-sensitivity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol. Microbiol. 8:521-524. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, Y., and A. Telenti. 2000. Genetics of drug resistance in Mycobacterium tuberculosis, p. 235-254. In G. Hatfull and W. R. Jacobs (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, DC.
- 21.Zhang, Y. 2004. Isoniazid, p. 739-758. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott Williams & Wilkins, New York, NY.
- 22.Zhang, Y., C. Vilcheze, and W. R. Jacobs. 2005. Mechanisms of drug resistance in Mycobacterium tuberculosis, p. 115-140. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus, 2nd ed. ASM Press, Washington, DC.