Fig. 1.
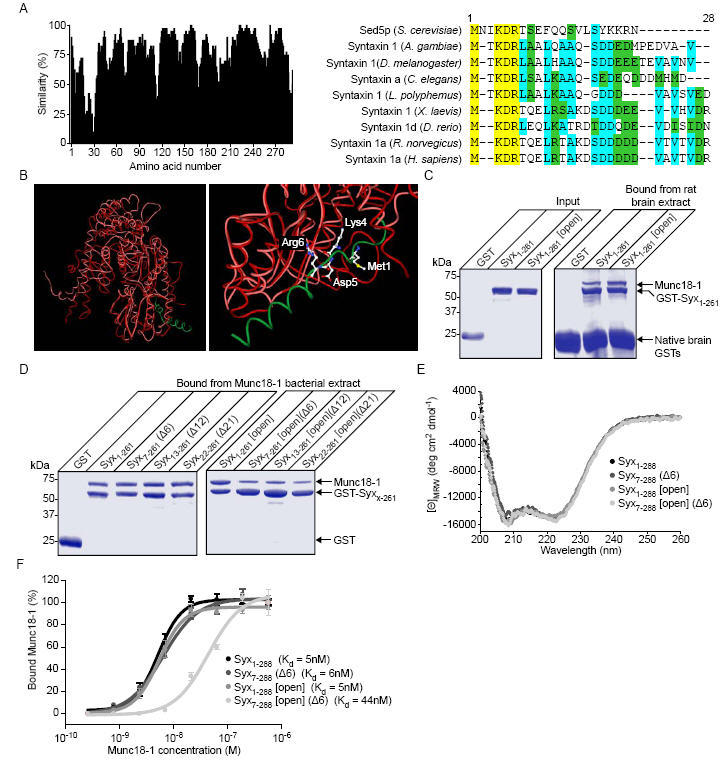
Syntaxin 1a can interact with munc18-1 through its N-terminus in vitro. A, Sequence alignment of syntaxin 1 homologues and Sed5p with similarity scored at each position (left panel). The highly conserved N-terminal region (a.a. 1- 28, right panel) is shown on a colour coded scale (yellow - identical, cyan-conserved, green - similar). B, Structural alignment of Sly1p (red) bound to an N-terminal peptide of Sed5p (green) (PDB: 1MQS(20)) and munc18-1 (pink) (PDB: 1EPU(39)). Highlighted on an enlarged view (right panel) are the absolutely conserved residues from panel a. C, Both Syx1-261 and Syx1-261 [open], immobilised on Sepharose beads, readily bind munc18-1. Native brain GSTs bound directly to the Sepharose resin. D, Truncation of the N-terminus of Syx1-261 [open] reduces its ability to bind munc18-1. N-terminal truncations of GST-Syx1-261 and GST-Syx1-261 [open] were immobilised on glutathione Sepharose beads and incubated with His6-munc18-1 containing bacterial lysate. Bound material was analysed by SDS PAGE and Coomassie staining. E, Circular dichroism spectra of Syx1-261, Syx1-261 (Δ6), Syx1-261 [open] and Syx1-261 [open] (Δ6). F, Measurement of the equilibrium dissociation constant for the syntaxin 1a-munc18-1 complex. GST-Syx1-261, or a mutant form, immobilised on Sepharose beads, was incubated in the presence of varying concentrations of munc18-1. Bound material was analysed by Western immunoblotting. Error bars represent S.E.M. (n=3).
