Figure 1.
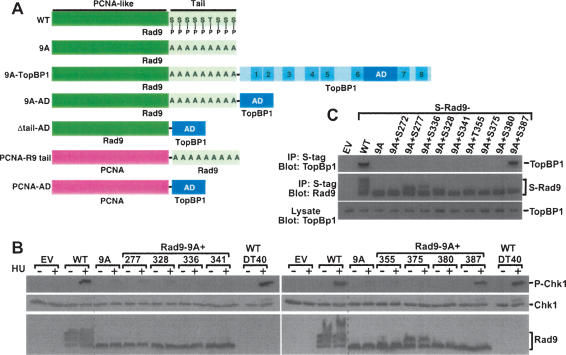
Rad9 Ser387 is involved in Chk1 phosphorylation. (A) Constructs used in this study. Rad9 and TopBP1 are not drawn to scale. Rad9 PCNA-like N-terminal domain and tail are indicated. P in tail indicates intact phosphorylation sites, whereas A indicates phosphorylation sites mutated to Ala. BRCT domains in TopBP1 are indicated; AD is the activation domain. (B) Rad9−/− DT40 cells were transiently transfected with empty vector (EV) and vectors encoding untagged wild-type Rad9 (WT); Rad9-9A (9A), the mutant lacking nine C-terminal phosphorylation sites; and Rad9-9A to which the indicated phosphorylation sites were restored (denoted as Rad9-9A + site). Following treatment with 10 mM HU for 1 h, transfected Rad9−/− DT40 cells and parental (wild-type) DT40 cells were lysed, separated by SDS-PAGE, and sequentially immunoblotted for phospho-Ser345-Chk1, Chk1, and Rad9. The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). The dotted line indicates the juxtaposition of nonadjacent regions of the same gel. (C) Lysates from HEK293 cells transiently transfected with empty vector (EV) or vectors encoding S-tagged wild-type Rad9 (WT), Rad-9A (9A), or the indicated Rad9-9A add-back expression plasmids were precipitated with S-protein agarose beads. Bound proteins were sequentially immunoblotted for endogenous TopBP1 (top) and Rad9 (middle). The multiple bands present in the Rad9 immunoblots are due to various forms of phosphorylated Rad9 (Volkmer and Karnitz 1999). (Bottom) A portion of the lysate was also immunoblotted to demonstrate equal TopBP1 levels in all samples.