Abstract
Epstein-Barr virus-associated hemophagocytic syndrome (EBV-AHS) is often associated with fatal infectious mononucleosis. However, the animal model for EBV-AHS has not been developed. We reported the first animal model for EBV-AHS using rabbits infected with EBV-related herpesvirus of baboon (HVP). Eleven of 13 (85%) rabbits inoculated intravenously with HVP-producing cells developed fatal lymphoproliferative disorders (LPD) between 22 and 105 days after inoculation. LPD was also accompanied by hemophagocytic syndrome (HPS) in nine of these 11 rabbits. The peroral spray of cell-free HVP induced the virus infection with increased anti-EBV-viral capsid antigen-IgG titers in three of five rabbits, and two of these three infected rabbits died of LPD with HPS. Autopsy revealed hepatosplenomegaly and swollen lymph nodes. Atypical lymphoid T cells expressing EBV-encoded small RNA-1 infiltrated diffusely in many organs, frequently involving the lymph nodes, spleen, and liver. Hemophagocytic histiocytosis was observed in the lymph nodes, spleen, bone marrow, and thymus. HVP-DNA was detected in the tissues and peripheral blood from the infected rabbits by polymerase chain reaction or Southern blot analysis. Reverse transcriptase-polymerase chain reaction revealed both HVP-EBNA1 and HVP-EBNA2 transcripts, suggesting latency type III infection. These data indicate that the high rate of rabbit LPD with HPS induction is caused by HVP. This system is useful for studying the pathogenesis, prevention, and treatment of human EBV-AHS.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus and a member of the γ herpesvirus family (lymphocryptovirus). Throughout the last 30 years, it has become well known that EBV is the etiological agent of acute infectious mononucleosis and is closely associated with the genesis of Burkitt’s lymphoma and nasopharyngeal carcinoma. The range of EBV-associated diseases has recently expanded to include not only various T, B, or NK cell lymphomas, Hodgkin’s disease, lymphoproliferative disorders (LPDs) of primary and secondary immunodeficiency, smooth muscle tumors, and gastric carcinoma, 1-3 but also EBV-associated hemophagocytic syndrome (EBV-AHS). 4-9
Hemophagocytic syndrome (HPS) is a systemic lymphohistiocytic proliferative disorder associated with infections, hematological malignancies, and X-linked LPDs (XLP or Duncan syndrome). 4-12 HPS is characterized by a systemic activation of macrophages that are induced to undergo phagocytosis. Chemokines play an important role in the recruitment of inflammatory cells into the tissues. Infection-associated hemophagocytic syndrome is usually associated with virus infections, especially EBV and other herpes group viruses, and is referred to as virus-associated hemophagocytic syndrome (VAHS). VAHS has been thought to be a distinct clinical entity, characterized by high fever, liver dysfunction, coagulation abnormalities, and pancytopenia. The demonstration of lymphohistiocytic infiltration with phagocytosis of erythrocytes and nucleated blood cells in the bone marrow, lymph nodes, spleen, and liver establishes the diagnosis of HPS. 6 EBV is now thought to be one of the major causes of this unique syndrome. 5-9 Spontaneous recovery from VAHS is common, but EBV-AHS is often associated with fatal infectious mononucleosis, and the prognosis for EBV-AHS is poor. 9,13 On the other hand, a potentially fatal hemophagocytic syndrome has also been noted in patients with malignant lymphomas (MLs), particularly in EBV-infected T-cell lymphoma. 4,7,14 Although EBV-AHS in previously healthy children or young adults is usually considered a reactive process, the clonal cytogenetic abnormalities that can emerge should be considered a malignant entity and treated with more intensive chemotherapy. 6,7,15-17 Many cases of hemophagocytic syndrome have been associated with viral infections, particularly EBV, but the pathogenesis of the syndrome remains unclear. The exact nature of EBV-AHS, ie, either an infectious process or a neoplastic disease, as well as the role of EBV, remains to be clarified.
Old World primates are naturally infected with a B-lymphotropic herpesvirus (gammaherpesvirus) closely related to EBV. These simian EBVs share considerable genetic, biological, and epidemiological features with human EBV, including virus-induced tumorigenesis. 18-22 These simian viruses can immortalize B lymphocytes. In addition, some simian EBV-like lymphocryptoviruses can also infect human B lymphocytes, 20,21,23 but are not usually associated with any known disease in natural host monkeys.
Herpesvirus papio (HVP) is a lymphocryptovirus from baboons that is similar to EBV both biologically and genetically. 19,21-28 The epidemiology of HVP infection in baboons closely parallels that of EBV infection in humans. 29 HVP can immortalize B lymphocytes from humans and various monkeys. Viral capsid antigen (VCA) of HVP appears similar to that of EBV, but most of the HVP-induced LCL lack a nuclear antigen analogous to EBV-associated nuclear antigen (EBNA) that can be detected by the anticomplement immunofluorescence tests. 24 HVP also has the potential to induce B cell LPD in the cotton-topped marmoset, a New World monkey. 24,25
We have previously reported an animal model of EBV-associated lymphomagenesis in humans: the malignant T-cell lymphoma induction of rabbits by EBV-like viruses from cynomolgus monkeys. 30-35 It was shown that malignant lymphomas frequently develop in ∼80 to 90% of rabbits after a short latency period (∼2 to 5 months) after intravenous injection or peroral spray of cynomolgus EBVs (Si-IIA-EBV, Cyno-EBV). This rabbit model was initially established by chance with studies of the HTLV-II-infected cynomolgus cell line (Si-IIA). 30,33,36
In the present study, we have established the first animal model of EBV-associated fatal HPS in which fatal LPD with HPS frequently develops in rabbits inoculated with HPV. A comparative analysis between this rabbit model and human EBV-AHS or EBV-associated lymphomas will be discussed herein.
Materials and Methods
Cells and Culture
An HVP-producing baboon lymphoblastoid cell line (594S) and a human EBV-producing marmoset cell line (B95-8) were used. As a control, normal baboon peripheral blood leukocytes that had been cultured with a complete medium for a short time were used.
Inoculation of Cells and Cell-Free Virion Pellets from Culture Supernatants
Specific pathogen-free normal New Zealand White rabbits (2 to 3 kg in weight) obtained from Shimizu Laboratory Supplies (Kyoto, Japan) were inoculated intravenously with 1.0 × 10 6 to 1.0 × 10 7 594S or control cells. All of the cells were lethally irradiated (100 Gy, γ irradiation) before inoculation, and the effectiveness of the irradiation was verified by the failure of the cells to grow in culture. They were also inoculated intravenously or orally sprayed with the cell-free virion pellets from 200 to 400 ml supernatants of a 594S culture, prepared as described below. Culture supernatants obtained from 594S culture (5 × 10 5 cells/ml) were first centrifuged at 8,000 × g for 30 minutes to remove cell debris (Himac CR20; Hitachi, Tokyo, Japan) and then at 100,000 × g for 60 minutes to obtain the pellets (Hitachi Himac Centrifuge SCP85H). These pellets were stocked in a freezer at −80°C until the experiments were performed. The stocked pellets from the supernatant (3,400 ml) were mixed and then divided into 12 crude virus fractions (each fraction was consistent with pellets from the 200-ml or 400-ml supernatant) (Table 1) ▶ .
Table 1.
Summary of Inoculations, Survival Times, and Pathological Findings in the Rabbits Inoculated with Herpesvirus Papio (Baboon EBV)
| Rabbit name | Inoculum | Route | Anti-VCA-IgG titer (days after inoculation) | Survival after inoculation (days) | LPD | Hemophagocytosis | Hepatosplenomegaly (liver necrosis) |
|---|---|---|---|---|---|---|---|
| N305 | 594S cell, 6×106 | iv | 40 (23) | 28 | + | + | + (+) |
| N306 | 594S cell, 6×106 | iv | 160 (23) | 28 | + | − | + (+) |
| N307 | 594S cell, 6×106 | iv | 320 (23) | 56 | + | + | + (−) |
| N308 | 594S cell, 6×106 | iv | 640 (24) | 43 | + | + | + (+) |
| N309 | 594S cell, 6×106 | iv | 640 (24) | 38 | + | + | + (+) |
| N310 | 594S cell, 6×106 | iv | 160–2560 (24–190) | 190 | − | − | − (−) |
| N311 | 594S cell, 6×106 | iv | 320 (24–48) | 75 | + | + | + (+) |
| N312 | 594S cell, 6×106 | iv | 320–640 (24–48) | 105 | + | + | + (+) |
| N317 | 594S cell, 1×107 | iv | N.E. | 22 | + | + | + (+) |
| N318 | 594S cell, 1×107 | iv | N.E. | 22 | + | + | + (+) |
| N335 | 594S cell, 1×106 | iv | 80 (28) | 37 (k) | + | + | + (−) |
| N336 | 594S cell, 1×106 | iv | 160 (28) | 95 | + | − | + (−) |
| N337 | 594S cell, 1×106 | iv | 320 (28) | 180 (k) | − | − | − (−) |
| N321 | Pellets of 594S supe (400 ml) | oral | 10–160 (28–39) | 51 | + | + | + (+) |
| N322 | Pellets of 594S supe (400 ml) | oral | 40–1280 (49–78) | 81 | + | + | + (+) |
| N323 | Pellets of 594S supe (400 ml) | oral | 80–1280 (28–81) | 81 (k) | − | − | − (−) |
| N324 | Pellets of 594S supe (400 ml) | oral | <10 (28–190) | 190 (k) | − | − | − (−) |
| N325 | Pellets of 594S supe (400 ml) | oral | <10 (28–190) | 190 | − | − | − (−) |
| N326 | Pellets of 594S supe (200 ml) | iv | 2560 (21) | 26 | + | + | + (+) |
| N327 | Pellets of 594S supe (200 ml) | iv | 640 (21) | 21 | + | + | + (+) |
| N328 | Pellets of 594S supe (200 ml) | iv | 2560 (21) | 23 | + | + | + (+) |
| N329 | Pellets of 594S supe (200 ml) | iv | 1280 (21) | 22 | + | + | + (−) |
| N330 | Pellets of 594S supe (200 ml) | iv | 1280 (21) | 22 | + | + | + (+) |
| N338 | Pellets of 594S supe (200 ml) | iv | 2560 (21) | 26 (k) | + | + | + (+) |
| N341 | Pellets of 594S supe (200 ml) | iv | 640 (21) | 28 (k) | + | + | + (−) |
| N(3) | B95-8 cell, 1×107 | iv | 80–320 (105) | 185 (k) | − (0/3) | − | − (−) |
| N(3) | normal PBL from baboon, 5×106 | iv | <10 (120) | 120 (k) | − (0/3) | − | − (−) |
LPD, lymphoproliferative disorder; VCA, viral capsid antigen of EBV; supe, supernatant; iv, intravenous; k, killed; N.E., not examined.
Antibody Responses to VCA of EBV or HVP and Laboratory Examination in Rabbits
The titers of anti-VCA-IgG in pre- and postinoculation-reserved sera from rabbits were retrospectively examined by an indirect immunofluorescence test using the P3HR-1 cell line as a standard antigen of VCA and fluorescein isothiocyanate-labeled goat anti-rabbit IgG (Cappel, West Chester, PA) as a secondary antibody.
In some rabbits, laboratory examination of rabbit peripheral blood was performed on the complete blood cell counting, glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), and lactate dehydrogenase (LDH).
Morphological Examination
Tumor-bearing rabbits appearing ill were killed with excess pentobarbital sodium (Abbott Laboratories, North Chicago, IL). All of the remaining animals were sacrificed by the 190 days postinoculation observation period. The organs including the spleen, liver, lymph nodes, lungs, thymus, kidneys, bone marrow, heart, and gastrointestinal tract were examined macroscopically and microscopically.
Phenotypic Analysis of HVP-Induced LPD
Tissues samples from rabbit LPD induced by 594S (HVP) inoculation were immunostained by the avidin-biotin-peroxidase complex (ABC) method (Immuno Mark Biotin Avidin Universal Kit; ICN Biochemical, Costa Mesa, CA) or the peroxidase anti-peroxidase (PAP) method using antibodies to rabbit CD45, CD5, CD4, MHC class II DQ (Serotec, Oxford, England), CD8, CD25 (Spring Valley Laboratories Inc., Woodbine, MD), RT1, RT2 (antibodies to rabbit T cells; Cedarlane Laboratories, Ontario, Canada), or RABELA (rabbit bursal equivalent to lymphocyte antisera, Cedarlane Laboratories), or human CD79a (DAKO, Kyoto, Japan).
Detection of EBV Genome in 594S Cells and 594S-Induced Rabbit LPD
EBV-Encoded Small RNA-1 (EBER-1) Expression
The EBV RNA in situ hybridization was performed using a single-stranded 30-base fluorescein isothiocyanate-labeled oligonucleotide complementary (anti-sense probe) or anti-complementary (sense, negative control probe) to a portion of the EBER-1 gene. The sequence of the anti-sense probe was 5′AGACACCGTCCTCACCACCCGGGACTTGTA-3′. The in situ hybridization was performed as described previously on routinely processed sections of the paraffin-embedded samples of 594S cells, and of the 594S-induced LPD lesions using the DAKO in situ hybridization kit. 31,32
Polymerase Chain Reaction (PCR)
The 594S cells (HVP) as the positive control, B95-8 (EBV), Ts-B6 (Cyno-EBV) cells, R424Spl (Cyno-EBV-induced rabbit lymphoma) and the spleen or peripheral blood from normal rabbits as the negative controls, and tissues from HVP-induced rabbit LPD lesions as the samples were digested at 37°C for 2 days with proteinase K (500 mg/ml) in digestion buffer (10 mmol/L Tris-HCl, 10 mmol/L ethylenediaminetetraacetic acid, 0.1% sodium dodecyl sulfate). DNA was extracted by the phenol/chloroform method and ethanol precipitation. Thirty cycles of PCR were performed on 50 ng of DNA in 50 μl of PCR mixture, which consisted of 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, 2.5 mmol/L MgCl2, 0.02% porcine gelatin (Sigma, St. Louis, MO), 200 nmol/L dNTPs, 200 mmol/L primer pair, and 2.5 U of Ex Taq polymerase (Takara, Kyoto, Japan). One primer pair for HVP-EBNA-1 and two primer pairs for HVP-EBNA-2 were used according to previous publications. 26,27 1) HPNA-1S: 5′CTGGGTTGTTGCGTTCCATG 3′, HPNA-1A: 5′TTGGGGGCGTCTCCTAACAA 3′; 2) HPNA2–1231S: 5′ACCACTGGGACCAGTTTGGT 3′, HPNA2–1612A: 5′AGAGGACTGAGGTTCTTGC 3′; 3) HPNA2–1485S: 5′AGCCTAGGCCCAATAGCTCA 3′, HPNA2–1691A: 5′CCTCCCATTGGTTGTCAGGG 3′. Amplified PCR products were electrophoresed in 3% NuSieve gel and visualized with 0.5 mg/ml ethidium bromide.
Southern Blot Analysis
The 594S (HVP) as the positive control, tissues from HVP-induced rabbit LPD lesions as the samples, B95-8 (EBV), Ts-B6 (Cyno-EBV) cells, and R424Spl (Cyno-EBV-induced rabbit lymphoma) as comparative controls, and Ra-1 (HTLV-I-transformed rabbit T -cell line), BALL-1, and normal rabbit spleens as the negative controls, were examined by Southern blotting for the presence of the EBV or HVP genome. The details of this procedure have been described elsewhere. 31,32 Briefly, DNAs (10 μg each) were digested by the restriction enzymes, XhoI, PstI, BamHI, and/or BglII (Bethesda Research Laboratories, Rockville, MD), subjected to electrophoresis in 0.8% agarose gel, and transferred and immobilized onto nylon membranes. Digested DNAs were hybridized with the EBV-BamHI W (3.1 kb), EBV-LMP1 (3.0 kb), EBV-XhoI (1.9 kb) fragment probes, or the HVP-EcoRI G fragment (3.6 kb, a homologue to the Z, R, K fragment of EBV) probe labeled by a random priming procedure with fluorescein-11-dUTP at 60°C overnight, washed three times, and detected using a chemiluminescence detection kit (Gene Images random prime labeling and detection system; Amersham Life Science, Buckinghamshire, England). For a clonality analysis of HVP, a purified PCR product of HVP DNA (nucleotide sequence: 5 to 608, NCBI GenBank Accession: AF200364) amplified by the primer pair: 5′ TCAATTCACAAGTCCTGGCG 3′ and 5′ CGTCCCATTAACTACCTCCA 3′ was used as a probe. This probe is specific to the region flanking terminal repeats of the virus and has been designated as HVPTR2.
Detection of EBNAs or mRNA of HVP-EBNAs and HVP-Latent Membrane Protein 1 (LMP1)
An indirect immunofluorescence test was also performed for the detection of EBNA1 and EBNA2 of human EBV. Smeared lymphoblastoid cell line (LCL, a positive control), Ra-1 (a negative control), 594S, Ts-B6, and 594S-induced LPD cells were fixed in acetone for 15 minutes at room temperature and incubated for 1 hour at 37°C with mouse monoclonal antibodies against EBV-EBNA1 (OT1x; kindly donated by Dr. J. Middeldorp), EBV-EBNA2 (DAKO, Kyoto, Japan), and LMP1 (CS1–4, DAKO). After being washed three times with PBS, the slides were incubated with fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (DAKO) and Hoechst 33258 for 30 minutes.
Reverse transcriptase (RT)-PCR was performed to detect the mRNA of HVP-EBNA1, HVP-EBNA2, and HVP-LMP1. Total RNA of HVP-induced rabbit LPD lesions, the positive control (594S), and the negative control (normal rabbit peripheral blood leukocytes) were extracted using the RNAqueous-Midi total RNA isolation kit (Ambion, Austin, TX). RT-PCR was performed with primer pairs: 1) HPNA-1S and 9869A: 5′ CTGCCCTTCCTCACCCTCAT 3′ for HVP-EBNA1; 2) HPNA2–1231S and HPNA2–1612A for HVP-EBNA2; 3) PLM173S: 5′ CTCACCTTAGCGCTTCTTGT 3′ and PLM362A: 5′ GCAATGAAGAGGACGAGCCA 3′ for HVP-LMP1, using mRNA selective PCR kit (Takara, Kyoto, Japan) containing AMV reverse transcriptase XL and AMV-optimized Taq.
Results
Incidence of LPD with VAHS in Rabbits Inoculated with 594S Cells or Cell-Free Virion Pellets
The pathological findings for the rabbit experiments are summarized in Table 1 ▶ . Of the 13 rabbits inoculated intravenously with HVP-producing simian 594S cells, 11 rabbits (85%) died of LPD 22 to 105 days after inoculation. LPD was also accompanied by VAHS in nine of these 11 rabbits. The peroral inoculation of HVP succeeded in the virus infection in three of five rabbits, and two of these three infected rabbits died of LPD with VAHS (51 to 81 days). LPD with VAHS was also induced in seven of seven rabbits (100%) by an intravenous injection of the cell-free pellets obtained from 594S culture supernatants 21 to 28 days after inoculation. As a whole, three infected rabbits had no LPD. Two rabbits without seroconversion after the peroral inoculation showed no abnormalities.
No lesions were detected in the six rabbits inoculated with normal peripheral blood leukocytes of baboon or EBV-producing cells (B95-8) during the period 120 to 185 days after inoculation.
Antibody Responses to VCA of EBV and Laboratory Data
All of the sera from rabbits inoculated intravenously with 594S (HVP) or B95-8 showed increased anti-EBV-VCA IgG antibody titers (×40 to ×2,560). In contrast, the pre-experimental sera and the sera from the other control rabbits were negative. However, an increase in anti-VCA-IgG antibodies was detected in only three of the five rabbits inoculated perorally with cell-free pellets from 594S culture supernatants (Table 1) ▶ .
Some rabbits with LPD and VAHS showed the elevation of GOT (∼116 IU/L), GPT (∼109 IU/L) and LDH (∼1,557 IU/L), and leukocytosis (∼21,500/mm3) with a mild increase of atypical lymphocytes (1 to ∼10%). A transient leukopenia (3,700 to ∼5,400/mm3) was also found in four of 10 rabbits examined.
Pathological Findings of Rabbits Inoculated with HVP (594S Cells)
Macroscopic Characteristics of the Infected Rabbits
The majority of the rabbits injected with 594S cells or cell-free pellets from a 594S culture appeared physically normal, except for anorexia and emaciation, but showed severe rhinorrhea admixed with blood and dyspnea during the few days before death. The necropsy of the infected rabbits revealed dark purple, swollen lymph nodes in the neck, mediastinum, axilla, mesentery, para-stomach, hepatic hilus, or inguinal regions (Figure 1A) ▶ . Mild or marked splenomegaly (Figure 1B) ▶ and/or hepatomegaly were usually observed. White nodules were sometimes found in the cross-section of the spleen, liver, or heart. The thymus appeared normal. Lungs showed congestion and edema, often accompanied by pulmonary hemorrhage. The three infected rabbits without LPD after inoculation with 594S cells or pellets from 594S culture looked ill transiently and then recovered later. Their autopsy revealed no macroscopic abnormalities.
Figure 1.
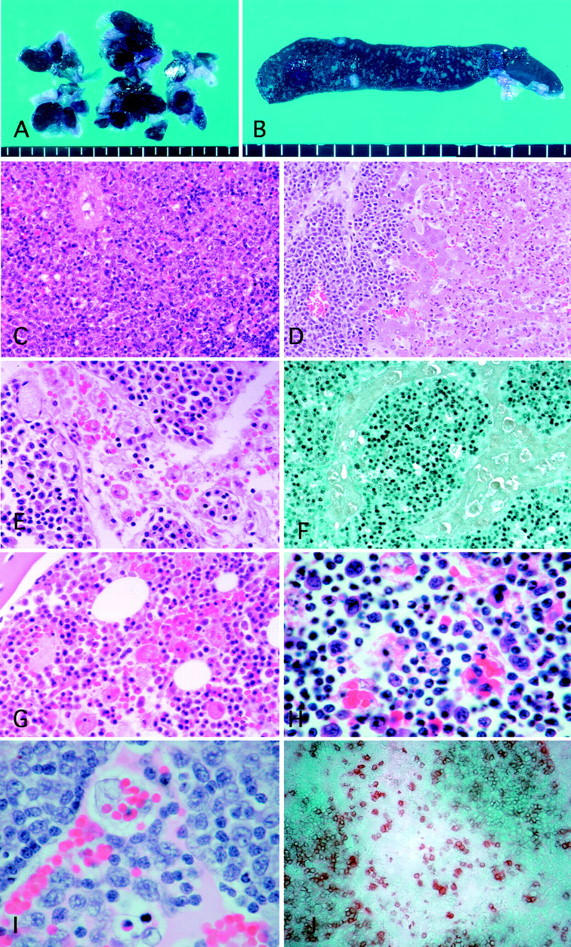
A: A marked swelling of dark purple lymph nodes with severe hemophagocytosis. B: Cross-section of the enlarged spleen showing small white nodular lesions. C: Diffuse infiltration of atypical large lymphoid cells in the spleen. D: Periportal infiltration of atypical large lymphoid cells and marked central necrosis of the hepatic lobule. E: The lymph node showing diffuse infiltration of atypical lymphoid cells and marked erythrophagocytosis in the sinus. F: Atypical lymphoid cells with EBER1 expression diffusely infiltrated the parenchyma of the lymph node with severe sinus hemophagocytosis. G: Hemophagocytosis in the bone marrow. H Infiltrated atypical lymphocytes and hemophagocytic cells with multiple ingested cell debris, lymphocytes, and erythrocytes observed in the thymus. I: High-power view of the lymph node showing atypical large lymphoid cell infiltration in both sinus and medulla. J: Marked infiltration of rabbit CD5-positive lymphoid cells in the lymph node. Original magnifications: ×150 (hematoxylin and eosin; C and D), ×300 (hematoxylin and eosin; E and G), ×750 (hematoxylin and eosin; H and I), ×150 (EBER1 in situ hybridization; F), and ×200 (rabbit CD5; J).
Microscopic Characteristics of 594S-Induced Rabbit LPD
The histological examination of rabbit tissues revealed mild to severe infiltration of atypical lymphoid cells involving many organs. Atypical large- or medium-sized lymphoid cells without Hodgkin’s cell-like morphology infiltrated around the perivascular areas with a diffuse or nodular pattern. The basic structure of the infiltrated organs was usually preserved but was sometimes destroyed in the area with a marked infiltration of atypical cells. Apoptotic cells (individual cell necrosis) accompanied by histiocytes containing cellular debris were sometimes observed in the atypical cell-infiltrated lesions. The lymph nodes, spleen, and liver were frequently and markedly involved (Figure 1C) ▶ . The involved livers showed severe periportal and sinusoidal infiltration of atypical lymphoid cells, often accompanied by central necrosis of hepatic lobules (Figure 1D) ▶ . Mild to moderate infiltration of atypical lymphoid cells was often observed in the kidneys, heart, and lungs. Most involved lymph nodes showed a diffuse infiltration of atypical lymphoid cells and marked hemophagocytosis in the sinus (Figure 1, E and I) ▶ . The expansion of interfollicular zone and disappearance of follicles were often observed but nodal sinus structures were usually not effaced except the lymph nodes with severe atypical lymphoid cell infiltration. Extramedullary hematopoiesis with megakaryocytic cells was seen occasionally in the sinus of lymph nodes admixed with atypical lymphoid cells. Focal infiltration of atypical lymphoid cells was found in the thymus or bone marrow in some cases. Atypical lymphoid cells also invaded the gastrointestinal tract, adrenal glands, tongue, salivary gland, and muscle. Atypical lymphocytes were often found in the blood vessels. Hemophagocytosis was also found in the spleen, bone marrow (Figure 1G) ▶ , and thymus (Figure 1H) ▶ .
Three rabbits that overcame the disease induced by the HVP showed no atypical lymphoid cell infiltration but did show severe hemosiderosis in the normal-sized spleen and scar-like lesions in the parenchymal organs, which must be the marks of infiltrated regions.
Immunophenotypes of the HVP-Induced Rabbit LPD
The immunohistochemical analyses of the atypical lymphoid cells revealed that atypical lymphoid cells were positive for rabbit CD45, CD5 (Figure 1J) ▶ , MHC class II DQ, CD25, RT1, and RT2, and negative for rabbit CD4, CD8, and RABELA. These atypical lymphoid cells showed no expression of CD79a (a human B-cell marker that cross-reacts with rabbit B cells).
Detection of the EBV Genome
EBER-1 Expression
The EBER-1 in situ hybridization revealed that most of the 594S cells expressed EBER-1. In 18 of 20 cases (90%) of LPD, EBER-1 expression was detected in virtually all atypical lymphoid cells (Figure 1F) ▶ . The EBER-1 signal was mostly nuclear. Rare and scattered small nonatypical lymphocytes with EBER-1 expression were also identified not only in some rabbits with LPD but in the rabbit (N323) that showed increased VCA titers but had no LPD at autopsy.
PCR
The PCR using two primer pairs for the HVP-EBNA-2 region (HPNA2-1231S and HPNA2-1612A: HPNA2-1485S and HPNA2-1691A) showed DNA amplification at the position of 382 bp or 207 bp, respectively, in the positive control (594S) and 594S (HVP)-induced rabbit LPD lesions (Figure 2A) ▶ . PCR using one primer pair for the HVP-EBNA-1 region (HPNA-1S and HPNA-1A) revealed amplified DNA at the position of 389 bp not only in the positive control (594S) and 594S (HVP)-induced rabbit LPD lesions but in the peripheral blood from the rabbits (N321, N322, N323, and N326 through N330) (data not shown). However, no amplification by PCR was seen in the negative controls [R424Spl (Cyno-EBV-induced tumor), Ts-B6 (Cyno-EBV), or B95-8 (human EBV)].
Figure 2.
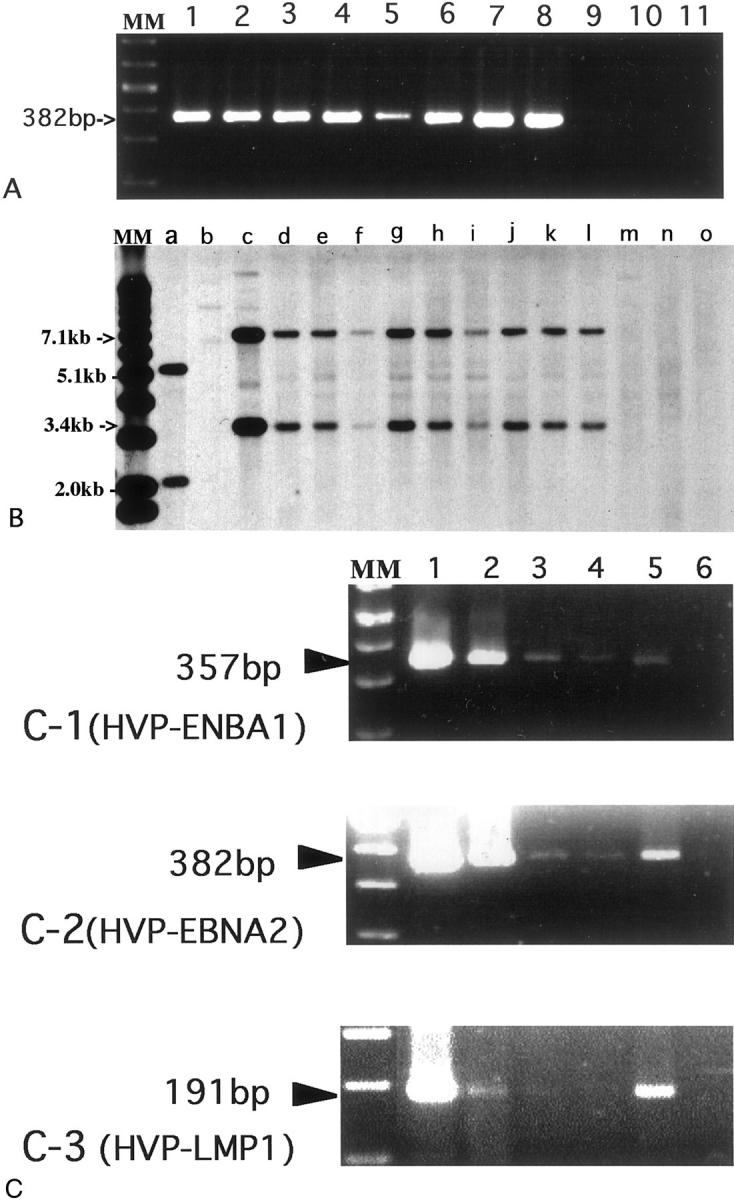
A: PCR for EBNA-2 of Herpesvirus papio (HVP) in HVP-induced LPD of rabbits. EBNA-2 DNA was amplified at the position of 382 bp in the positive control [lane 1, 594S (HVP)] and HVP-induced rabbit LPD lesions (lanes 2 through 8: N317LN, N317Liv, N317Spl, N317Thy, N318LN, N318Liv, N318Spl, respectively), but not in the negative controls [lane 9, R424Spl (Cyno-EBV-induced tumor), lane 10, Ts-B6 (Cyno-EBV), lane 11, B95-8 (human EBV)]. MM, molecular marker. B: Southern blot analysis for the presence of HVP DNA in HVP-induced LPD of rabbits. Sample DNAs were digested by BamHI and BglII, and hybridized with the HVP-EcoRI G fragment probe that includes the EBNA-1 region. Two positive bands (3.4 kb and 7.1 kb) were detected in the positive control (lane c, 594S) and in HVP-induced LPD of rabbits (lanes d through l: N328Spl, N328Liv, N328LN, N329Spl, N329Liv, N329kid, N330Spl, N330Liv, and N330LN, respectively). B95-8 (lane a) showed two different-sized positive bands. No positive band was detected in lane b (Ts-B6), lane m (R424Spl), lane n (Ra-1), or lane o (BALL-1). MM, molecular marker. C: RT-PCR revealed expression of both HVP-EBNA1 mRNA (C-1) and HVP-EBNA2 mRNA (C-2) in the positive control (lane 1, 594S) and in HVP-induced LPD of rabbits (lanes 2 through 5: N338Spl, N338LN, N341Spl, and N341LN, respectively), but not in the negative control (lane 6). The mRNA of HVP-LMP1 was detected in 594S, N338Spl and N341LN (C-3).
Southern Blot Analysis
The Southern blot analysis with HVP-EcoRI G fragment probe after DNA digestion by both BamHI and BglII revealed the presence of HVP DNA at the positions of 3.4 kb and 7.1 kb in the positive control (594S), 594S (HVP)-induced rabbit LPD lesions (N328Spl, N328Liv, N328LN, N329Spl, N329Liv, N329kid, N330Spl, N330Liv, N330LN) but not in Ts-B6 (Cyno-EBV), R424Spl (Cyno-EBV-induced lymphoma), or the negative control (Ra-1) (Figure 2B) ▶ . Two positive bands at the different-sized position were detected in B95-8 (Figure 2B) ▶ . The Southern blot analysis with the EBV-BamHI W probe, EBV-LMP1 probe, or EBV-XhoI 1.9-kb fragment showed positive bands only in B95-8, Ts-B6, or Ts-B6-induced lymphoma, but no positive band was detected in 594S and 594S-induced LPD lesions of the rabbits.
Clonality analysis using the HVPTR2 probe revealed monoclonal or oligoclonal bands in the 594S (HVP)-induced rabbit LPD lesions (data not shown).
Detection of EBNAs or mRNA of HPV-EBNAs and LMP1 in 594S Cells and 594S (HVP)-Induced Rabbit LPD Lesions
The indirect immunofluorescence study using anti-human EBV monoclonal antibodies revealed EBNA-1, EBNA-2, and LMP1 expression in the positive control cells (LCL) and B95-8, but no cross-reactivity of EBNA-1, EBNA-2, or LMP1 was detected in the 594S cells and 594S-induced rabbit lesions.
The mRNAs of HVP-EBNA1 and HVP-EBNA2 were detected by RT-PCR in 594S cells and HVP-induced rabbit LPD lesions but not in the negative control (Figure 2 ▶ , C-1 and C-2, respectively). HVP-LMP1 transcript was detected in two of four samples examined at the position of 191 bp (Figure 2 ▶ , C-3).
Discussion
EBV-AHS is a distinct disease characterized by high mortality. Rare cases with primary EBV infection develop fatal infectious mononucleosis, which is commonly accompanied by VAHS, whereas infectious mononucleosis is usually a self-limiting disease. 9
EBV-associated B cell proliferation with VAHS was observed in most cases of XLP, 9 each one case of sporadic or familial HPS 4 and a few cases of Japanese fatal LPD. 37 On the other hand, EBV-associated T-cell proliferation with a potentially fatal HPS has been noted not only in many cases of T-cell lymphoma 7 but in three Japanese and 15 Taiwanese patients with fatal childhood EBV-AHS, which were caused by LPD of primarily EBV-infected T cells. 6,7,38 EBV-associated natural killer cell proliferation with fatal VAHS has been known in a Vietnamese case. 15 The emergence of not only the monoclonal or biclonal proliferation but also clonal cytogenetic abnormalities of EBV-infected cells observed in Japanese and Taiwanese cases of fatal childhood EBV-AHS 16,39 should be considered malignant entities and treated with more intensive chemotherapy. A large series of cytogenetic and molecular studies is needed to clarify the exact nature of this fatal disease.
In the present study, atypical lymphocytes in the LPD of rabbits inoculated with HVP expressed EBER1 and were found by PCR or Southern blot analysis to contain HVP-DNA, but not human EBV nor cynomolgus EBV. In toto, the data described above indicate that the LPD and VAHS of rabbits were induced by HVP derived from 594S cells, but not by human EBV or other well-known simian oncogenic viruses such as cynomolgus EBV. The origin of atypical cells in rabbit LPD is not baboon cells but rabbit lymphoid cells, because both intravenous and peroral inoculation of cell-free HVP could induce LPD in rabbits and inoculated 594S baboon cells were lethally irradiated. This rabbit model of fatal LPD with VAHS induced by primary infection of HPV showed clinicopathological features similar to childhood EBV-AHS in that: 6,7 (1) the rabbits used were previously healthy and had no immunodeficiency background; (2) the affected rabbits showed seroconversion and a fulminant course and high mortality from anywhere from 3 weeks to 3 months, and hepatosplenomegaly with liver injury or necrosis, systemic lymph node swelling, and a bleeding tendency were frequently observed; (3) a typical lymphoid cell infiltration was detected in many organs, particularly the lymph nodes, spleen, and liver; (4) fatal rabbit LPD with HPS is induced by monoclonal or oligoclonal atypical T cell proliferation; (5) hemophagocytosis is also present in the lymph nodes (markedly), spleen (moderate), and bone marrow (mild); and (6) this rabbit system is also developed by oral spray of HVP, indicating that infection occurs by the same natural transmission route of human EBV.
The latency type of EBV has not yet been identified in fatal EBV-AHS in childhood. However, the latency type of HVP infection in rabbits with fatal LPD and VAHS is thought to be type III because all transcripts of HVP-EBNA1, HVP-EBNA2, and HVP-LMP1 have been detected in the rabbit lesions examined. There are no available antibodies specific or cross-reactive to HVP-EBNA1 or HVP-EBNA2. Therefore, we used the RT-PCR method to detect EBNA expression. However, the detection of type III latency by RT-PCR in HVP-induced rabbit LPD lesions does not necessarily mean that all HVP-infected cells have latency type III. In another words, it is possible that rabbit LPD lesions may contain not only type III but also type I or type II latency cells. If the nature of rabbit LPD were neoplastic, it would then be necessary to examine the latency type of the neoplastic cell lines from rabbit LPD lesions.
In the laboratory examination of rabbit peripheral blood, we confirmed the elevation of GOT, GPT, and LDH levels in some rabbits with LPD and VAHS. Leukocytosis with a mild increase in atypical lymphocytes and transient leukopenia in peripheral blood were also observed in some of the rabbits examined. However, pancytopenia was not detected. This may be explained by mild hemophagocytosis in rabbit bone marrow.
Cytokine elevations are one of the characteristics of HPS, including elevations in interleukin (IL)-1β, IL-3, macrophage colony-stimulating factor, IL-6, interferon-γ, prostaglandin, and tumor necrosis factor-α levels. Recent evidence indicates that the pathophysiology in EBV-AHS seems to be mediated by EBV-infected T cells selectively releasing tumor necrosis factor-α, which, in combination with interferon-γ and probably other cytokines, can activate macrophages. 7,8,40 The rabbit cytokine detection system has not been established yet. However, analysis of rabbit cytokine levels in this animal model is very important and needed.
We have tabulated a comparative overview analysis, presented in Table 2 ▶ , of EBV-associated lymphomas in human, 41 simian EBV-associated lymphoma in rabbits, 31-35 and HVP-induced rabbit LPD with VAHS. The rabbit fatal LPD with VAHS model induced by HVP presented in this study is comparable to human fatal infectious mononucleosis with VAHS (fatal EBV-AHS in childhood), which has been suggested to be T-cell malignant lymphoma based on recent accumulated evidence of its clonal cytogenetic abnormalities. 7,15-17 Further studies are needed to clarify the true nature of this rabbit fatal LPD with VAHS model: that is, are the proliferating lymphoid cells neoplastic or reactive? Do they have clonal cytogenetic abnormalities or not? The oligoclonal or monoclonal expansion of rabbit lymphoid cells in rabbit LPD in vivo was observed by Southern blot analysis on EBV termini, suggesting their neoplastic character. In contrast, the death of rabbits with HVP infection was usually caused by VAHS, which involved a tendency to bleed, especially terminal hemorrhage of lungs, and liver damage as well as LPD. In preliminary trials, we failed to establish cell lines from rabbits with HVP-induced LPD, whereas cell lines were easily established from rabbit malignant lymphoma by cynomolgus EBV. 33 Characterization of the rabbit cell lines from HVP-induced rabbit LPD lesions is essential to elucidate the true nature of these LPD lesions.
Table 2.
Comparative Overview of EBV-Associated Lymphomas in Humans and Simian EBV-Like Virus-Associated Diseases in Rabbits
| Lymphoma type | Phenotype | Typical latent period | EBV positivity | EBV gene expression | Latency type |
|---|---|---|---|---|---|
| Burkitt’s ML | B cell | 3–8 years | 100% (endemic), 15–85% (sporadic) | EBNA1+ | I |
| Opportunistic ML | B cell | <1 year post-transplant, 5–10 years post-HIV70–80% | 100% | EBNA1+, EBNA2+, LMP1+ | III |
| Fatal IM | B cell | <6 months | 100% | EBNA1+, EBNA2+, LMP1+ | III |
| PAL | B cell | 33 years post-treatment | 100% | EBNA2+, LMP1+ | III |
| VAHS/fatal IM (T-cell ML) | T cell | <6 months | 100% | ? | |
| T-cell ML (AILD/pleomorphic) | T cell | >30 years | 40%? | EBNA2−, LMP1+ | II |
| Nasal T/NK cell ML | T/NK cell | >30 years | 100% | EBNA1+, LMP1+/− | II |
| Hodgkin’s disease | >10 years | 39–90% | EBNA1+, LMP1+ | II | |
| Rabbit ML model by cynomolgus EBV | T cell | 2–5 months | 100% | EBNA1+, EBNA2−, LMP1−? | I |
| Rabbit fatal LPD with VAHS model by baboon EBV (HVP) | T cell | 3 weeks–3 months | 100% | HVP-EBNA1 transcript+, HVP-EBNA2 transcript+, HVP-LMP1 transcript+ | III |
This table was modified from the table in the textbook. 41
ML, malignant lymphoma; PAL, pyothorax-associated lymphoma; VAHS, virus-associated hemophagocytic syndrome; IM, infectious mononucleosis; AILD, angioimmunoblastic lymphadenopathy-like; LPD, lymphoproliferative disorders; HVP, Herpesvirus papio.
The reasons for the high susceptibility of rabbits to LPD with VAHS by HVP remain to be elucidated. If the pathogenic mechanism of human VAHS were the same as that of this rabbit model, we can speculate that HVP-infected proliferating T cells secrete a nonregulated excess of cytokines, which activate macrophages and induce a storm of cytokine production from macrophages, resulting in VAHS. The immunogenic virus-related antigens recognized by cytotoxic T cells may not be expressed on some of the HVP-infected proliferative lymphoid cells or, alternatively, the cytotoxic T-cell response may be disturbed. 42 Further studies, including a characterization of rabbit cell lines established from HVP-induced LPD and in vitro transformation experiments of rabbit lymphocytes, are needed to address these problems.
Animal models of EBV infection have been reported in some New World primates (cotton-top marmosets or tamarins), which have developed lymphoproliferative disease after EBV inoculation. 41,42 However, the value of the tamarin model as a more general model for EBV infections in humans is limited by the inability to infect these animals via the natural oropharyngeal route and/or virus persistence in animals.
Each of the Old World primate species is commonly infected with a different, but closely related lymphocryptovirus. This common evolutionary origin with EBV strongly suggests that the essential features of the virus-host interaction will have been conserved in primates. 18 Moghaddam and colleagues 43 have demonstrated that the pathogen-free rhesus monkey orally infected with EBV-like virus from rhesus monkeys is a good animal model for acute and persistent infection of human EBV. An EBV-like herpesvirus (HVMF1) isolated from lymphomas of SIV-infected cynomolgus monkeys has been identified as a causative agent for a monkey model of EBV-associated lymphomagenesis in human AIDS. 44,45 The other EBV-related viruses from cynomolgus monkeys (Cyno-EBV, Si-IIA-EBV) can induce a high rate of rabbit T-cell lymphomas. 31-35 The sequence analysis of the IR1 (BamHIW) or EBNA1 region of cynomolgus EBVs (HVMF1, Cyno-EBV, and Si-IIA-EBV) indicates that these regions of cynomolgus EBVs are highly similar to each other and suggests that Cyno-EBV, Si-IIA-EBV, and HVMF1 may be variants of each other. 33,46 However, there has previously been no animal model for EBV-AHS, and the rabbit LPD with VAHS induced by HVP examined in the present study represents the first animal model.
In conclusion, the direct causative relationship between HVP infection in rabbits and the subsequent LPD and VAHS development in the infected rabbits is very clear in this experimental model. In view of the scarcity of nonhuman primates and their expense, this rabbit model is a useful and inexpensive alternative experimental model for studying the biology and pathogenesis of EBV, especially in relation to human EBV-related fatal LPD and VAHS.
Acknowledgments
We thank the late Prof. Kanji Hirai, Tokyo Medical and Dental University, for his kind donation of EBV probes; Ms. Reiko Endo, Ms. Yoshiko Sakamoto, Ms. Hiromi Nakamura, Ms. Rika Hayashi, Ms. Mutsumi Okabe, and Ms. Miyuki Shiotani, Department of Pathology, Okayama University Medical School; and Mr. Hiroshi Okamoto and Ms. Chizuru Motochika, the Central Research Laboratory, Okayama University Medical School.
Footnotes
Address reprint requests to Kazuhiko Hayashi, M.D., Second Department of Pathology, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama-city 700-8558, Japan. E-mail: kazuhaya@med.okayama-u.ac.jp.
Supported by grant 11470058 from the Ministry of Education, Science, Sports, and Culture, Japan.
References
- 1.Weiss LM, Chang KL: Association of the Epstein-Barr virus with hematolymphoid neoplasia. Adv Nat Pathol 1996, 3:1-15 [Google Scholar]
- 2.Anagnostopoulos I, Hummel M: Epstein-Barr virus in tumours. Histopathology 1996, 29:297-315 [DOI] [PubMed] [Google Scholar]
- 3.Kawa K: Epstein-Barr virus-associated diseases in humans. Int J Hematol 2000, 71:108-117 [PubMed] [Google Scholar]
- 4.Gaffey MJ, Frierson HF, Jr, Medeiros LJ, Weiss LM: The relationship of Epstein-Barr virus to infection-related (sporadic) and familial hemophagocytic syndrome and secondary (lymphoma-related) hemophagocytosis: an in situ hybridization study. Hum Pathol 1993, 24:657-667 [DOI] [PubMed] [Google Scholar]
- 5.Su IJ, Hsu YH, Lin MT, Cheng AL, Wang CH, Weiss LM: Epstein-Barr virus-containing T-cell lymphoma presents with hemophagocytic syndrome mimicking malignant histiocytosis. Cancer 1993, 72:2019-2027 [DOI] [PubMed] [Google Scholar]
- 6.Su IJ, Chen RL, Lin DT, Lin KS, Chen CC: Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol 1994, 144:1219-1225 [PMC free article] [PubMed] [Google Scholar]
- 7.Su IJ, Wang CH, Cheng AL, Chen RL: Hemophagocytic syndrome in Epstein-Barr virus-associated T-lymphoproliferative disorders: disease spectrum, pathogenesis, and management. Leuk Lymphoma 1995, 19:401-406 [DOI] [PubMed] [Google Scholar]
- 8.Kikuta H: Epstein-Barr virus-associated hemophagocytic syndrome. Leuk Lymphoma 1995, 16:425-429 [DOI] [PubMed] [Google Scholar]
- 9.Okano M, Gross TG: Epstein-Barr virus-associated hemophagocytic syndrome and fatal infectious mononucleosis. Am J Hematol 1996, 53:111-115 [DOI] [PubMed] [Google Scholar]
- 10.Risdall RJ, Mckenna RW, Nesbit ME, Krivit W, Balfour HH, Jr, Simons RL, Brunning RD: Virus-associated hemophagocytic syndrome. A benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 1979, 44:993-1002 [DOI] [PubMed] [Google Scholar]
- 11.Mroczek EC, Weisenburger DD, Grierson HL, Markin R, Purtilo DT: Fatal infectious mononucleosis and virus-associated hemophagocytic syndrome. Arch Pathol Lab Med 1987, 111:530-535 [PubMed] [Google Scholar]
- 12.Favara BE: Hemophagocytic lymphohistiocytosis: a hemophagocytic syndrome. Semin Diagn Pathol 1992, 9:63-74 [PubMed] [Google Scholar]
- 13.Ohshima K, Shimazaki K, Sugihara M, Haraoka S, Suzumiya J, Knada M, Kawasaki C, Kukuchi M: Clinicopathological findings of virus-associated hemophagocytic syndrome in bone marrow: association with Epstein-Barr virus and apoptosis. Pathol Int 1999, 49:533-540 [DOI] [PubMed] [Google Scholar]
- 14.Craig FE, Clare CN, Sklar JL, Banks PM: T-cell lymphoma and the virus-associated hemophagocytic syndrome. Am J Clin Pathol 1992, 97:189-194 [DOI] [PubMed] [Google Scholar]
- 15.Dolezal MV, Kamel OW, van de Rijn M, Cleary ML, Sibley RK, Warnke RA: Virus-associated hemophagocytic syndrome characterized by clonal Epstein-Barr virus genome. Am J Clin Pathol 1995, 103:189-194 [DOI] [PubMed] [Google Scholar]
- 16.Chen JS, Tzeng CC, Tsao CJ, Su WC, Chen TY, Jung YC, Su IJ: Clonal karyotype abnormalities in EBV-associated hemophagocytic syndrome. Haematologica 1997, 82:572-576 [PubMed] [Google Scholar]
- 17.Ito E, Kitazawa J, Arai K, Otomo H, Endo Y, Imashuku S, Yokoyama M: Fatal Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis with clonal karyotype abnormality. Int J Hematol 2000, 71:263-265 [PubMed] [Google Scholar]
- 18.Kieff E: Epstein-Barr virus and its replication. Fields BN Knipe DM Howley PM eds. Fields Virology. 1996, :pp 2343-2396 Lippincott-Raven, Philadelphia [Google Scholar]
- 19.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F: Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol 1996, 70:7819-7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillner J, Rabin H, Letvin N, Henle W, Henle G, Klein G: Nuclear DNA-binding proteins determined by the Epstein-Barr-virus-related simian lymphotropic herpesviruses H. gorilla, H. pan, H. pongo and H. papio. J Gen Virol 1987, 68:1587-1596 [DOI] [PubMed] [Google Scholar]
- 21.Heller M, Gerber P, Kieff E: DNA of herpesvirus Pan, a third member of the Epstein-Barr virus-herpesvirus papio group. J Virol 1982, 41:931-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Tanaka A, Lau RY, Nonoyama M, Rabin H: Linkage map of the fragments of herpesvirus papio DNA. J Virol 1981, 37:710-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moghaddam A, Koch J, Annis B, Wang F: Infection of human B lymphocytes with lymphocryptoviruses related to Epstein-Barr-virus. J Virol 1998, 72:3205-3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk LA: A review of Herpesvirus papio, a B-lymphotropic virus of baboons related to EBV. Comp Immun Microbiol Infect Dis 1979, 2:257-264 [DOI] [PubMed] [Google Scholar]
- 25.Deinhardt F, Falk L, Wolfe LG, Schudel A, Nonoyama M, Lai P, Lapin B, Yakovleva L: Susceptibility of marmosets to Epstein-Barr virus-like baboon herpesvirus. Prim Med 1978, 10:163-170 [PubMed] [Google Scholar]
- 26.Ling PD, Ryon JJ, Hayward SD: EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol 1993, 67:2990-3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yates JL, Camiolo SM, Ali S, Ying A: Comparison of the EBNA1 proteins of Epstein-Barr virus and herpesvirus papio in sequence and function. Virology 1996, 222:1-13 [DOI] [PubMed] [Google Scholar]
- 28.Fuentes-Panana EM, Swaminathan S, Ling PD: Transcriptional activation signals found in the Epstein-Barr virus (EBV) latency C promoter are conserved in the latency C promoter sequences from baboon and Rhesus monkey EBV-like lymphocryptoviruses (cercopithicine herpesviruses 12 and 15). J Virol 1999, 73:826-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenson HB, Ench Y, Gao SJ, Rice K, Carey D, Kennedy RC, Arrand JR, Mackett M: Epidemiology of herpesvirus papio infection in a large captive baboon colony: similarities to Epstein-Barr virus infection in humans. J Infect Dis 2000, 181:1462-1466 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Ohara N, Koirala TR, Ino H, Chen HL, Teramoto N, Kondo E, Yoshino T, Takahashi K, Yamada M, Tomita N, Miyamoto K, Fujimoto K, Yoshikawa Y, Akagi T: HTLV-II non-integrated malignant lymphoma induction in Japanese white rabbits after intravenous inoculation of HTLV-II-infected simian leukocyte cell line (Si-IIA). Jpn J Cancer Res 1994, 85:808-818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi K, Koirala T, Ino H, Chen H-L, Ohara N, Teramoto N, Yoshino T, Takahashi K, Yamada M, Nii S, Miyamoto K, Fujimoto K, Yoshikawa Y, Akagi T: Malignant lymphoma induction in rabbits by intravenous inoculation of Epstein-Barr-virus-related herpesvirus from HTLV-II-transformed cynomolgus leukocyte cell line (Si-IIA). Int J Cancer 1995, 63:872-880 [DOI] [PubMed] [Google Scholar]
- 32.Hayashi K, Chen H-L, Yanai H, Koirala TR, Ohara N, Teramoto N, Oka T, Yoshino T, Takahashi K, Miyamoto K, Fujimoto K, Yoshikawa Y, Akagi T: Cyno-EBV (EBV-related herpesvirus from cynomolgus macaques) induces rabbit malignant lymphomas and their tumor cell lines frequently show specific chromosomal abnormalities. Lab Invest 1999, 79:823-835 [PubMed] [Google Scholar]
- 33.Hayashi K, Akagi T: An animal model for Epstein-Barr virus (EBV)-associated lymphomagenesis in the human: malignant lymphoma induction of rabbits by EBV-related herpesvirus from cynomolgus. Pathol Int 2000, 50:85-97 [DOI] [PubMed] [Google Scholar]
- 34.Koirala TR, Hayashi K, Chen H-L, Ino H, Kariya N, Yanai H, Choudhury CR, Akagi T: Malignant lymphoma induction of rabbits with oral spray of Epstein-Barr virus-related herpesvirus from Si-IIA cells (HTLV-II-transformed Cynomolgus cell line): a possible animal model for Epstein-Barr virus infection and subsequent virus-related tumors in humans. Pathol Int 1997, 47:442-448 [DOI] [PubMed] [Google Scholar]
- 35.Chen H-L, Hayashi K, Koirala TR, Ino H, Fujimoto K, Yoshikawa Y, Choudhury CR, Akagi T: Malignant lymphoma induction in rabbits by oral inoculation of crude virus fraction prepared from Ts-B6 cells (Cynomolgus B-lymphoblastoid cells harboring Epstein-Barr virus-related simian herpesvirus). Acta Med Okayama 1997, 51:141-147 [DOI] [PubMed] [Google Scholar]
- 36.Hayashi K, Ohara N, Fujiwara K, Aoki H, Jeon HJ, Takahashi K, Tomita N, Miyamoto K, Akagi T: Co-expression of CD4 and CD8 associated with elevated interleukin-4 in a cord T cell line derived by coculturing normal human cord leukocytes and an HTLV-II-producing simian leukocyte cell line (Si-IIA). J Cancer Res Clin Oncol 1993, 119:137-141 [DOI] [PubMed] [Google Scholar]
- 37.Yatabe Y, Mori N, Oka K, Iijima T, Saga S, Takada K, Asai J: Fatal Epstein-Barr virus-associated lymphoproliferative disorder in childhood. Arch Pathol Lab Med 1995, 119:409-417 [PubMed] [Google Scholar]
- 38.Kawaguchi H, Miyashita T, Herbst H, Niedobitek G, Asada M, Tsuchida M, Hanada R, Kinoshita A, Sakurai M, Kobayashi N, Mizutani S: Epstein-Barr virus-infected T lymphocytes in Epstein-Barr virus-associated hemophagocytic syndrome. J Clin Invest 1993, 92:1444-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuta H, Sakiyama Y, Matsumoto S, Oh-Ishi T, Nakano T, Nagashima T, Oka T, Hironaka T, Hirai K: Fatal Epstein-Barr virus-associated hemophagocytic syndrome. Blood 1993, 82:3259-3264 [PubMed] [Google Scholar]
- 40.Lay JD, Tsao CJ, Chen JY, Kadin ME, Su IJ: Upregulation of tumor necrosis factor-alpha gene by Epstein-Barr virus and activation of macrophages in Epstein-Barr virus-infected T cells in the pathogenesis of hemophagocytic syndrome. J Clin Invest 1997, 100:1969-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickinson AB, Kieff E: Epstein-Barr virus. Fields Virology. Edited by BN Fields, DM Knipe, PM Howley: Philadelphia, Lippincott-Raven, 1996, pp 2397–2446
- 42.Miller G, Shope T, Coope D, Waters L, Pagano J, Bornkamm GW, Henle W: Lymphoma in cotton-top marmosets after inoculation with Epstein-Barr virus: tumor incidence, histologic spectrum, antibody responses, demonstration of viral DNA, and characterization of viruses. J Exp Med 1977, 145:948-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson RP, Wang F: An animal model for acute and persistent Epstein-Barr virus infection. Science 1997, 276:2030-2033 [DOI] [PubMed] [Google Scholar]
- 44.Feichtinger H, Li SL, Kaaya EE, Putkonen P, Grunewald K, Weyrer K, Bottinger D, Ernberg I, Linde A, Bieberfeld G, Bieberfeld P: A monkey model for Epstein-Barr-virus associated lymphomagenesis in human acquired immunodeficiency syndrome. J Exp Med 1992, 176:281-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezikyan S, Kaaya EE, Ekman M, Voevodin AF, Feichtinger H, Putkonen P, Castanos-Velez E, Bieberfeld G, Bieberfeld P: B-cell lymphomagenesis in SIV-immunosuppressed cynomolgus monkeys. Int J Cancer 1995, 61:574-579 [DOI] [PubMed] [Google Scholar]
- 46.Ohara N, Hayashi K, Teramoto N, Oka T, Fujimoto K, Yoshikawa Y, Castanos-Velez E, Biberfeld P, Akagi T: Sequence analysis and variation of EBNA-1 in Epstein-Barr virus-related herpesvirus of cynomolgus monkey. Intervirology 2000, 43:102-106 [DOI] [PubMed] [Google Scholar]


