Figure 1.
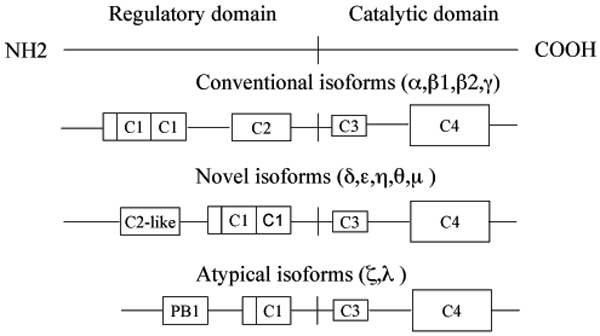
Diagrammatic representation of the domain structure of PRKC isoforms. Conventional, cPRKC isoforms (PRKCA, PRKCB1, PRKCB2, and PRKCG also known as PRKCα,β1,β2, and γ, respectively) are diacylglycerol (DAG) sensitive and Ca2+ responsive (C2 is the Ca2+-binding domain, C1 features the DAG and phosphatidylserine binding; at the N-terminal of the C1 domain is located a pseudosubstrate site that regulates PRKC activity). Novel, nPRKC isoforms (PRKCD, PRKCE, PRKCH, PRKCQ, and PRCKM also known as PRKC δ,ε,η,θ, and μ, respectively) are DAG sensitive but Ca2+ insensitive (the C2-like domain do not retain Ca2+-coordinating residues). Atypical, aPRKC isoforms (PRKCZ and PRKCI, also known as PRKC ζ and λ, respectively) are regulated by phospholipidic mediators products including phosphatidylinositol 3,4,5-triphosphate). All kinases have a conserved kinase core (C4) and a C3 domain, which represents the ATP-binding site (for a review see [12].
