Abstract
Gestational and early postnatal iron deficiency occurs commonly in humans and results in altered behaviors suggestive of striatal dysfunction. We hypothesized that early iron deficiency alters the metabolome of the developing striatum and accounts for abnormalities in striatum-dependent behavior in rats. Sixteen metabolite concentrations from a 9-11 μL volume within the striatum were serially assessed in 10 iron-deficient and 10 iron-sufficient rats on postnatal days 8, 22 (peak anemia), and 37 (following recovery from anemia) using 1H NMR spectroscopy at 9.4 tesla. Chin-elicited bilateral forelimb placing and vibrissae-elicited unilateral forelimb placing were also assessed on these days. Iron deficiency altered metabolites indexing energy metabolism, neurotransmission, glial integrity, and myelination over time (P< 0.05). Successful development of behaviors was delayed in the iron-deficient group (P ≤ 0.01). Alterations in creatine, glucose, glutamine, glutamate, N-acetylaspartate, myoinositol, and glycerophosphorylcholine + phosphorylcholine concentrations accounted for 77-83% of the behavioral variability during peak anemia on postnatal day 22 in the iron-deficient group. Correction of anemia normalized the striatal metabolome but not the behaviors on postnatal day 37. These novel data imply that alterations in the metabolite profile of the striatum likely influence later neural functioning in early iron deficiency.
Introduction
The most common cause of gestational and early postnatal iron deficiency in the world is mothers who are iron-deficient (ID)11 during the periods of pregnancy and lactation. Anemia, potentially due to iron deficiency, complicates most pregnancies in developing countries (1). Gestational diabetes mellitus and intrauterine growth restriction also place the fetus at risk for brain iron deficiency (2,3). Despite iron replenishment, chronically ID infants continue to have suboptimal performance on motor and mental measures and more behavioral problems than infants who were never ID (1,4-7). There is also evidence that neurocognitive alterations may persist into adolescence (8). Many of these behaviors have been linked to dysfunction of the striatum, a brain region that develops rapidly during the late gestational and early postnatal periods. The striatum is activated in the presence of stimuli associated with reward and in the presence of aversive, novel, unexpected, or intense stimuli. Perturbations in the nigrostriatal tracts and dopamine metabolism are associated with changes in motor control, altered perception, memory, and motivation consistent with behavioral changes seen in ID children (4-11).
Rodent models of early iron deficiency support the hypothesis that some behavioral changes are associated with short- and long-term alterations in striatal dopamine homeostasis (12,13). The behavioral abnormalities include delayed appearance of forelimb placing reflexes elicited by chin or vibrissae stimulation, incomplete automatic grooming chains, increased hesitancy, and decreased exploratory behavior. Associated dopaminergic changes include increased extracellular concentration of dopamine and its metabolites and increased dopamine transporter levels in the striatum.
The effects of early iron deficiency are not limited to changes in brain monoamine neurotransmitter metabolism and include glutamate metabolism, energy metabolism, and myelin production (14-16). These metabolite alterations in developing brain regions can be assessed simultaneously and longitudinally using 1H NMR spectroscopy at high magnetic fields (e.g., 9.4 tesla) during typical development and under adverse perinatal conditions, such as perinatal iron deficiency and chronic hypoxia (16,17). The effects of gestational and early neonatal iron deficiency on the striatal metabolome and the relation of these metabolite changes to striatum-dependent behaviors are not yet known.
The objective of the present study was to evaluate the effects of gestational and lactational iron deficiency on 1) the metabolome of the striatum during its development using high-field 1H NMR spectroscopy, and 2) the acquisition of 2 striatum-dependent behaviors, chin-elicited bilateral forelimb placing (also known as head-on) and vibrissae-elicited unilateral forelimb placing, that emerge postnatally in rats (18,19). Furthermore, we hypothesized that there would be an association between changes in metabolites involved in energy, neurotransmitter and myelination pathways, and delays in striatum-dependent behaviors.
Materials and Methods
Animals
Experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee of the University of Minnesota. Pregnant Sprague Dawley dams (Harlan, Sprague Dawley) were received on gestational day (G) 3 and were housed in the local animal facility. The number of pups in a litter was randomly culled to 8 on postnatal day (P) 3-4, keeping the male:female ratio equal as much as possible. Rats had free access to food and water and were maintained on a 12-h light:dark cycle at room temperature.
Study design
Pregnant dams were randomly assigned, upon arrival, to either an ID diet (ID group) or iron-sufficient (IS) diet (IS group). A total of 8 litters (4 IS and 4 ID) were raised. Our dietary model was designed to replicate the phenomenon of mothers who are ID during pregnancy and remain deficient throughout the lactation period. The low-iron diet (Formula TD80396, Harlan-Teklad; elemental iron concentration: 3-6 mg/kg) was administered from G3 to P7, followed by a slightly iron-supplemented diet (Formula TD01094, Harlan-Teklad; elemental iron concentration: 10 mg/kg) from P7 until weaning on P22, when an iron-supplemented diet (Formula TD89300, Harlan-Teklad; elemental iron concentration: 50 mg/kg) was started and continued until the end of the experiment on P37. The dams and pups, after weaning, were fed the iron-supplemented diet (50 mg/kg) from G3 to P37 in the IS group. The composition of diet TD80396 has been previously published (20). The other 2 diets were prepared by adding 0.03 mg of ferrous sulfate (FeSO4 · 7H20)/kg diet (TD 01094) or 0.235 mg of ferrous citrate/kg diet (TD 89300) to this diet. Analysis of the diets using atomic absorption spectroscopy method demonstrated an iron concentration (μg/g) of 2.86 (TD80396), 9.38 (TD01094), and 48.50 (TD89000), respectively.
A total of 53 rats (7-11/group on P8, P22, and P37) from 7 litters (3 IS, 4 ID) were utilized for brain tissue iron assay and hematocrit. Twenty rats (10 IS and 10 ID) from 4 litters (2 IS and 2 ID) were longitudinally studied by 1H NMR spectroscopy and performed striatum-dependent behaviors on P8, P22, and P37. Two rats in the IS group died during the experimental period, one on P15 and the other on P37. Littermates of same sex and similar body weight were substituted on both occasions. There was no mortality in the ID group.
1H NMR spectroscopy
All experiments were performed on a 9.4 tesla/31 cm magnet (Magnex Scientific) interfaced to a Varian INOVA console (Varian). Spontaneously, breathing rats were anesthetized by flow of a gas mixture (N2O:O2 = 1:1) containing 1.5-2% isoflurane. The air temperature surrounding the rats was maintained at 30°C by warm-water circulation and verified by a thermosensor. The duration of a study of a single animal did not exceed 100 min. The detailed technical aspects of the 1H NMR spectroscopy have been described previously (16,17,21,22). Positioning of the volume of interest within the striatum in the sagittal and coronal planes was based on multislice rapid acquisition with relaxation enhancement imaging (echo train length = 8, echo spacing = 15 ms, field of view = 2 × 2 cm, matrix = 256 × 256, slice thickness = 1 mm). The volume of interest (9-11 μL) was adjusted to match the postnatal increase in striatal size between P8 and P37.
Quantification of metabolites
In vivo 1H NMR spectra were analyzed using the LCModel (23) as in our previous studies (16,17). The signals of macromolecules and the following 16 metabolites were quantified from each spectrum: alanine, ascorbate, aspartate, creatine, γ-aminobutyric acid (GABA), glucose, glutamate, glutamine, glutathione, lactate, myoinositol, N-acetylaspartate (NAA), N-acetylaspartylglutamate, phosphocreatine, phosphorylethanolamine, and taurine. Unsuppressed water signal was used as an internal reference for the quantification of metabolites, assuming a brain water content of 88, 82, and 79% on P8, P22, and P37, respectively, based on a previous study (16). Absolute concentrations of the metabolites were expressed as μmol/g tissue. Due to a strong cross-correlation in the quantification of similar spectra, the sum of glycerophosphorylcholine (GPC) and phosphorylcholine (PCho) (GPC + PCho), was determined. The phosphocreatine:creatine and glutamate:glutamine ratios were also analyzed. Thus, the metabolome consisted of 17 metabolites and 2 metabolite ratios.
Behavioral assessments
Both chin-elicited bilateral forelimb placing and vibrissae-elicited unilateral forelimb placing were assessed during the rat’s light cycle on P8, P22, and P37 before anesthesia was initiated for NMR spectroscopy. For bilateral forelimb placing, the rat was held by its torso and the ventral aspect of the chin was placed in contact with the horizontal surface of a table. A successful response was determined by the immediate and simultaneous placing of both forelimbs on the table surface. For unilateral forelimb placing, the rat was held by its torso, allowing one forelimb to hang free. The vibrissae on the ipsilateral side were brushed against the table edge. A successful response was determined by the immediate placing of the ipsilateral forelimb on the table surface. A score of 1 was given for each successful trial in both behavioral tests. Each rat performed 10 sequential comfortable trials of each behavior on each day. Unilateral forelimb placing has been traditionally used to study the effects of unilateral stroke in rats on contralateral limb placing (19). Since iron deficiency does not preferentially affect one hemisphere over the other, the means of the right and left placing results were determined at each age. Group means were determined for both behavioral tasks.
Biochemical analysis
Rats were deeply anesthetized with sodium pentobarbital administered intraperitoneally. Blood was collected by cardiac puncture for determining the hematocrit. The animals were transcardially perfused with phosphate buffered saline (pH 7.4) until the effluent was clear. Following decapitation, the brain was removed and frozen at -80°C. Brain iron concentrations were assayed by atomic absorption spectroscopy, as previously described (24). Values were expressed as μmol of elemental iron/g wet tissue.
Statistical analysis
Group means of body and brain weights and hematocrit and tissue iron concentration between the 2 dietary groups were compared using ANOVA, and the differences at the 3 postnatal ages (i.e., on P8, P22, and P37), were determined using Bonferroni-adjusted two-tailed t tests. The hypotheses regarding the effect of postnatal age, effect of diet, and effect of interaction between age and diet (age × diet) on the metabolites and behaviors were tested with multivariate linear or generalized linear regression models (for behavioral outcomes). Generalized estimating equation methodology (25) was used to fit the linear or generalized linear models to the outcomes of metabolites and behaviors. We predicted that peak behavior changes between the ID and IS groups would occur concurrently with peak anemia, which occurred on P22. Therefore, we planned to perform a secondary analysis using a logistic regression model to determine whether the metabolite alterations on P8 or P22 could predict behavior on P22. Finally, we performed a multiple regression analysis to determine the magnitude of the variance in behavioral tests on P22 that was accounted for by the metabolites that differed between the 2 dietary groups by generating overall r and r2 values for each behavior. All statistical tests were two-tailed and significance level was set at P = 0.05, except for post hoc analysis, where significance level was set at P = 0.02 to correct for multiple comparisons. Statistical analyses were conducted using SAS version 9.1 (SAS Institute).
Results
General
The body weight and brain weight increased between P8 and P37 in both groups (Table 1). There was an effect of age on body and brain weights, hematocrit, and brain iron concentration (P < 0.02). Diet had an effect on all variables except the brain weight (P < 0.001), and age × diet affected all variables except the brain iron concentration (P < 0.001). The ID group had lower body weight and hematocrit than the IS group on P8 and P22 (P < 0.001). The brain iron concentration was 50% lower in the ID group on P8 (P < 0.003). This difference decreased to 25 and 15% on P22 and P37, respectively (Table 1).
TABLE 1.
Body weight, brain weight, hematocrit, and brain iron concentration of iron-sufficient and iron-deficient rats1
| Iron-sufficient |
Iron-deficient |
|||||
|---|---|---|---|---|---|---|
| P8 | P22 | P37 | P8 | P22 | P37 | |
| Body weight,2,3 g | 20.0 ± 1.0 | 72.0 ± 1.0 | 144.0 ± 5.0 | 15.0 ± 0.0* | 44.0 ± 0.0* | 102.0 ± 4.0 |
| Brain weight,3 g | 0.55 ± 0.02 | 1.16 ± 0.03 | 1.44 ± 0.08 | 0.54 ± 0.01 | 1.05 ± 0.06 | 1.40 ± 0.08 |
| Hematocrit2,3 | 0.38 ± 0.01 | 0.33 ± 0.01 | 0.40 ± 0.01 | 0.18 ± 0.00* | 0.12 ± 0.01* | 0.41 ± 0.01 |
| Brain Fe,2 μmol/g | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.03 ± 0.01* | 0.05 ± 0.01 | 0.06 ± 0.00 |
Values are means ± SEM, n =7–11. For all variables, age had an effect, P < 0.02.
Different from iron-sufficient group of the same age, P ≤ 0.003.
Diet had an effect, P < 0.001.
Age × diet had an effect, P < 0.001.
1H NMR spectroscopy
Developmental changes were evident in the 1H NMR spectra obtained on P8, P22, and P37 (Fig. 1). We assessed the role of postnatal age on the metabolite profile and confirmed a developmental trajectory for all metabolites in both dietary groups (P < 0.001; Table 2). Differences were significant between the ID and IS groups for the following metabolites: glucose, glutamine, GPC + PCho, myo-inositol, lactate, NAA, phosphocreatine, taurine, and glutamate:glutamine ratio (P < 0.05). There was an age × diet interaction on ascorbate, creatine, glucose, glutamate, myo-inositol, NAA, taurine (P < 0.05). Iron deficiency did not affect alanine, aspartate, glutathione, N-acetylaspartylglutamate, and phophorylethanolamine. The greatest number of metabolites was affected on P22, the time of peak anemia. Most alterations resolved by P37, when anemia had also resolved and brain iron concentration approached control values.
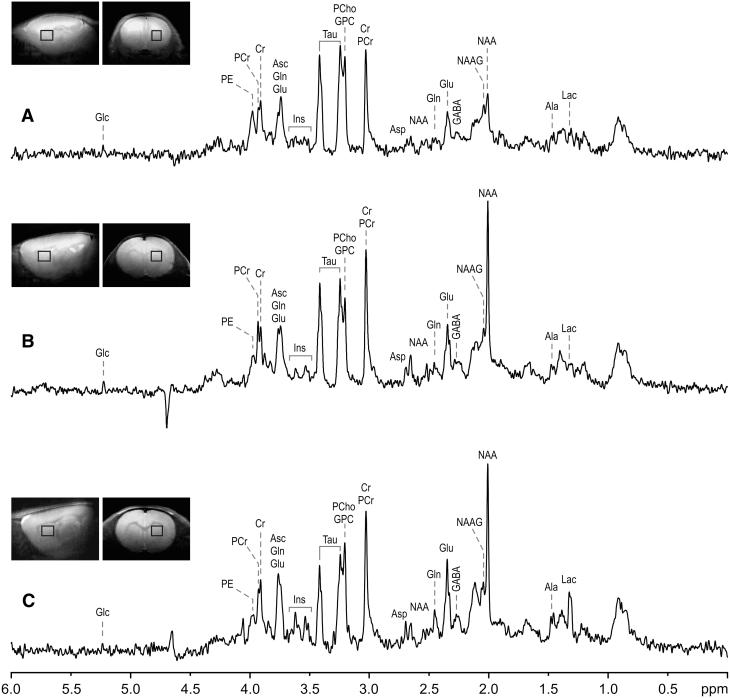
Figure 1.
Sagittal and coronal MRI, and 1H NMR spectra of the iron-deficient striatum. 1H NMR spectra were obtained from a 9-11 μL volume of the striatum (boxes in MRI) on P8 (A), P22 (B), and P37 (C). Ala, alanine; Asc, ascorbate; Asp, aspartate; Cr, creatine; GABA, γ-aminobutyric acid; Glc, glucose; Glu, glutamate; Gln, glutamine; GPC, glycerophosphorylcholine; Lac, lactate; Ins, myo-inositol; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PCr, phosphocreatine; PCho, phosphorylcholine; PE, phosphorylethanolamine; Tau, taurine.
TABLE 2.
Metabolite concentrations in the striatum of iron-sufficient and iron-deficient rats1
| Iron-sufficient |
Iron-deficient |
|||||
|---|---|---|---|---|---|---|
| Metabolite | P8 | P22 | P37 | P8 | P22 | P37 |
| μmol/g tissue | ||||||
| Ascorbate3 | 3.70 ± 0.17 | 2.48 ± 0.11 | 2.08 ± 0.11 | 3.95 ± 0.07 | 2.38 ± 0.05 | 2.40 ± 0.10 |
| Creatine3 | 3.21 ± 0.07 | 3.54 ± 0.09 | 3.44 ± 0.07 | 3.32 ± 0.08 | 3.20 ± 0.07* | 3.62 ± 0.08 |
| GABA3 | 0.87 ± 0.07 | 1.40 ± 0.03 | 1.35 ± 0.08 | 0.95 ± 0.08 | 1.30 ± 0.05 | 1.57 ± 0.07 |
| Glucose2,3 | 3.41 ± 0.14 | 1.97 ± 0.11 | 2.01 ± 0.20 | 3.76 ± 0.22 | 3.49 ± 0.24* | 2.12 ± 0.23 |
| Glutamate3 | 5.08 ± 0.12 | 9.73 ± 0.16 | 10.36 ± 0.12 | 5.87 ± 0.15* | 8.78 ± 0.26* | 10.63 ± 0.13 |
| Glutamine2 | 2.18 ± 0.11 | 2.47 ± 0.12 | 4.51 ± 0.18 | 2.03 ± 0.11 | 2.00 ± 0.10* | 3.98 ± 0.14 |
| GPC + PCho2 | 1.57 ± 0.06 | 1.27 ± 0.04 | 1.37 ± 0.03 | 1.67 ± 0.04 | 1.01 ± 0.03* | 1.32 ± 0.10 |
| Lactate2 | 1.08 ± 0.07 | 1.37 ± 0.09 | 3.41 ± 0.17 | 1.32 ± 0.08 | 1.80 ± 0.16 | 3.51 ± 0.15 |
| Myo-inositol2,3 | 1.33 ± 0.08 | 2.69 ± 0.09 | 3.76 ± 0.08 | 1.44 ± 0.04 | 1.91 ± 0.10* | 3.68 ± 0.09 |
| N-acetylaspartate2,3 | 2.56 ± 0.06 | 7.13 ± 0.07 | 7.67 ± 0.10 | 2.80 ± 0.06* | 7.57 ± 0.08* | 7.73 ± 0.09 |
| Phosphocreatine2 | 2.75 ± 0.08 | 3.65 ± 0.08 | 3.27 ± 0.05 | 2.98 ± 0.08 | 3.87 ± 0.10 | 3.34 ± 0.07 |
| Taurine2,3 | 11.40 ± 0.16 | 11.72 ± 0.09 | 8.98 ± 0.12 | 11.98 ± 0.16* | 11.72 ± 0.09 | 8.98 ± 0.12* |
| Glu:Gln ratio2 | 2.40 ± 0.14 | 4.04 ± 0.22 | 2.33 ± 0.09 | 3.00 ± 0.24 | 4.52 ± 0.33* | 2.70 ± 0.10 |
Values are means ± SEM, n =10. For all metabolites, age had an effect, P < 0.001.
*Different from iron-sufficient group of the same age (P < 0.02). GABA, γ aminobutyric acid; Glu:Gln, glutamate:glutamine; GPC 1 PCho, sum of glycerophosphorylcholine and phosphorylcholine.
Diet had an effect, P < 0.05.
Age × diet had an effect, P < 0.05.
Behavioral assessment
Successful performance of the 2 striatum-dependent behaviors increased over time in both groups (P < 0.001), confirming a developmental trajectory (Table 3). The IS group had the greatest improvement between P8 and P22, with full development of the task by P37. The ID group, however, had <50% success on both behaviors on P22 and continued to be significantly less successful on P37, despite correction of anemia. The ID group had delayed appearances of both behaviors, resulting in a significant age × diet interaction (P = 0.01 for bilateral forelimb placing and P = 0.002 for averaged left and right unilateral forelimb placing;=Table 3).
TABLE 3.
Successful performance of striatum-dependent behaviors by iron-sufficient and iron-deficient rats1
| Iron-sufficient |
Iron-deficient |
|||||
|---|---|---|---|---|---|---|
| Behavioral test | P8 | P22 | P37 | P8 | P22 | P37 |
| Bilateral forelimb placing | 2 ± 0.5 | 9 ± 0.4 | 10 ± 0.0 | 0 ± 0.0 | 5 ± 0.8* | 9 ± 0.2* |
| Unilateral forelimb placing | 0 ± 0.0 | 7 ± 0.5 | 10 ± 0.2 | 0 ± 0.0 | 3 ± 0.6* | 8 ± 0.4* |
Values are means ± SEM, n = 10. For both behavioral tests, significant effects of age, P < 0.001; diet (P < 0.001); age × diet, P ≤ 0.01.
Different from iron-sufficient group of the same age, P < 0.01.
Based on peak behavioral differences between groups on P22, multiple regression analysis was used to assess to what degree the metabolites that were significantly different between the dietary groups on P22 accounted for the variance in behavior at that time point. Striatal concentrations of creatine, glucose, glutamate, glutamine, NAA, myo-inositol, and GPC + PCho on P22 accounted for 77-83% of the variance in the behavioral performance (bilateral forelimb placing: r = 0.88, r2 = 0.77; mean unilateral forelimb placing: r = 0.91, r2 = 0.83).
Logistic regression analysis was used to assess whether antecedent metabolite changes on P8 (i.e., period of peak brain iron deficiency) predicted behavior on P22. Overall, there were few metabolites on P8 that predicted abnormal behavior on P22. Specifically, the model predicted that the probability of successful performance on bilateral forelimb placing on P22 decreased if rats were ID on P8 or had higher striatal concentrations of GPC + PCho, lactate, or glutamate:glutamine ratio on P8. Only lactate and glutamate:glutamine ratio were higher in the ID group on P8. The model predicted that the probability of successful performance on mean unilateral forelimb placing on P22 decreased if rats were ID or had higher concentrations of glutamine or GPC + PCho on P8. None of the P8 metabolite difference between the diet groups predicted the unilateral behavioral retardation of the ID group on P22.
A similar analysis assessed whether concurrent metabolite changes on P22 predicted behavioral impairment on P22. The probability of successful performance on bilateral forelimb placing decreased if rats had higher striatal concentrations of glutamine, NAA, or GPC + PCho or lower concentrations of phosphorylethanolamine, phosphocreatine, or myo-inositol on P22. NAA was indeed higher and myo-inositol was lower in the ID group on P22, both results are consistent with less-successful performance. The model also predicted that the probability of successful performance on unilateral forelimb placing decreased if rats were ID or had lower concentrations of GPC + PCho or phosphorylethanolamine on P22. None of the metabolite difference between dietary groups on P22 predicted the unilateral behavioral retardation of the ID group on P22. Overall, hierarchical regression demonstrated that alterations in 2 metabolites measured in the striatum, NAA, and myo-inositol, were best related to the decreased behavioral performance during iron deficiency.
Discussion
This study examined the consequences of combined gestational and lactational iron deficiency on the metabolome of the developing striatum and on striatum-dependent behaviors. The dietary model produced changes in the metabolome, most of which were resolved by P37 with iron replenishment. The concurrent metabolite changes correlated with and accounted for the delayed acquisition of striatum-dependent behaviors. Yet, despite the correction of the striatal metabolome with iron treatment and the resolution of anemia, behavioral abnormalities persisted.
The metabolomic analysis demonstrated that, although the overall developmental trajectories of metabolites were similar in the 2 dietary groups, multiple metabolites were altered in the striatum due to iron deficiency. Whereas some of the metabolite changes were similar to those demonstrated in the ID hippocampus reported from our laboratory previously (16), changes in others, especially those on P22, were similar to those described for the ID hippocampus exposed to chronic hypoxia (17). Taken together, this suggests that brain iron deficiency, combined with potential hypoxia due to anemia, is likely responsible for the metabolomic alterations in the striatum of the ID group.
The metabolite changes demonstrate that markers of energy metabolism (phosphocreatine and creatine), energy substrates (glucose and lactate), amino acids, neurotransmitters (glutamate, γ-aminobutyric acid, and taurine), and markers of neuronal and glial integrity and myelination (glutamine, myo-inositol, and NAA) are altered in the striatum due to gestational and lactational iron deficiency. Alterations in multiple metabolites suggest that the effect of iron deficiency is pervasive and likely involves multiple biochemical pathways in the developing striatum.
The ID rats in this study had a delayed acquisition of striatum dependent behaviors. Whether these behavioral impairments are permanent is not known, because the assessment did not extend beyond P37. Recent studies using the same dietary model showed similar delayed acquisition of these striatal tasks (12,13). These striatum-based behavioral changes were largely attributed to alterations in dopaminergic homeostasis based on an extensive literature demonstrating changes in dopamine concentration, receptor concentrations, and reuptake mechanisms in this dopamine-rich structure (9,18,19,26,27). Furthermore, the behavioral phenotypes of ID animals are thought to closely resemble animals with lesions to dopaminergic neurons (13,18,28,29).
This study showed that ∼80% of the variability in the 2 tested behaviors was accounted for by concurrent alterations in metabolite markers of energy metabolism, glutamatergic neurotransmission, glial integrity, and myelination. These processes were also implicated in prior whole brain and hippocampal iron deficiency models (14,16,30) but were not previously assessed in the striatum.
The phosphocreatine concentrations were, overall, higher in the ID group. This was accompanied by lower creatine concentrations on P22, resulting in an increased phosphocreatine:creatine ratio during the period of peak anemia. We propose that hypoxia due to severe anemia is responsible for the lower creatine in the ID striatum. This is supported by a similar decrease in creatine demonstrated in the hippocampus subjected to chronic hypoxia (17) and the normalization of creatine with correction of anemia on P37 in the present study. The increased phosphocreatine and decreased creatine suggests that energy production needed for cellular structure and function, including myelin production and synaptic transmission, may be impaired in the ID striatum (31-34).
As in our previous study of the hippocampus (16), iron deficiency altered glutamate and glutamine concentrations in the striatum so that the glutamate:glutamine ratio was increased. We postulate that increased glutamate:glutamine ratio represents suppressed glutamatergic neurotransmission in the ID striatum. Glutamate released from the neurons during glutamatergic neurotransmission is taken up by the adjacent astrocytes, converted to glutamine, and transferred back to the neurons, where it is reconverted to glutamate. Glutamate uptake and conversion to glutamine in the astrocytes are energy-demanding processes (35-37) that are likely to be compromised in the energy-limited ID striatum. As further discussed below, we propose that suppressed glutamatergic neurotransmission may be responsible for the altered dopamine metabolism demonstrated in the ID striatum, insofar as both neurotransmitters modulate each other’s action in the striatum (38). Finally, increased glutamate and decreased glutamine concentrations may also suggest the potential for increased vulnerability to oxidative injury in the ID striatum. Similar alterations in glutamate and glutamine were demonstrated in the striatum of mice lacking H-ferritin, a model of iron deficiency and oxidative stress (39).
The logistic regression model identified 2 concurrent metabolites, NAA and myo-inositol, related to the decreased behavioral performance during iron deficiency. In addition, multiple regression analysis demonstrated that the striatal concentrations of glutamine, NAA, myo-inositol, and GPC + PCho on P22 predicted 77-83% of the variance in the behavioral performance. This leads to the speculation that the striatal process most affected by gestational and lactational iron deficiency is oligodendroglial cell integrity and myelination, because the affected metabolites are markers of glial integrity and membrane phospholipid biosynthesis (40-46). Human and rat studies have shown that brain iron is vital for myelination (14,47-52). In addition, oligodendrocytes require iron for energy production and myelin synthesis (49).
Higher levels of striatal NAA in ID rats could represent a defect in deacetylation of NAA required during myelin production. During rapid postnatal brain growth, the peak uptake of brain iron coincides with the period of peak myelinogenesis. Human studies have found poorer motor control and altered auditory brainstem responses in formerly ID infants (8,50,51), both ascribed to impaired myelination. The results of our study may provide metabolomic evidence for a delay in myelin maturation.
Previous literature, including work from our own group, demonstrated that impairments in striatum-dependent behaviors in iron deficiency are associated with alterations in dopamine metabolism (12,13). In the present study, ∼80% of the observed behavioral changes from iron deficiency were attributable to metabolite alterations that did not include dopamine, because the latter cannot be measured by NMR spectroscopy. Without simultaneous assessment of dopaminergic metabolites, it is not possible to determine the relative roles of dopamine and nondopamine metabolites in accounting for these specific behavioral impairments, which are strongly driven by the monoamine system (13,18,19,29). As proposed earlier, it is possible that the glutamatergic changes we observed in the striatum and the hippocampus (16) interact with the striatal dopaminergic system. Extensive evidence demonstrates a complex interaction between the dopaminergic and glutamatergic systems in the striatum, nucleus accumbens, prefrontal cortex, and hippocampus (53-58). For example, the hippocampal glutamatergic system affects the tonic state of the dopamine neurons in the ventral tegmental area (55). Adequate glutamatergic hippocampal input to the striatum is important for maintaining striatal dopaminergic neurons in a potentiated state. With reduced neuronal output from the hippocampus (59), characterized by altered glutamatergic homeostasis (16), it is possible that changes in the glutamatergic system affect the likelihood with which striatal dopaminergic neurons fire (55). In this study and in the previous one (16), the neurotransmitter abnormalities on NMR spectroscopy appear as glutamatergic in the hippocampus (16) and glutamatergic input to the striatum; however, the resultant downstream behavioral effects in the striatum (12) appear to be dopaminergic.
In conclusion, iron deficiency induced metabolite changes that accounted for a large proportion of striatum-dependent behavioral changes. The observed changes were the ones expected to be affected by iron deficiency and included alterations in markers of energy metabolism, neurotransmission, neuronal and glial integrity, and myelination. Additional studies are necessary to determine whether these metabolite alterations are selective to the striatum or also occur in other brain areas in gestational and lactational iron deficiency. Hierarchically, changes in metabolite markers of myelination were the ones most prominently affected; however, the interaction of the glutamatergic and dopaminergic changes, as well as metabolite alterations that are not detected by conventional NMR methods, or other, as yet to be determined factors induced by brain iron deficiency, could also account for the observed behavioral abnormalities. Of potential clinical significance, correction of iron deficiency anemia by P37 normalized the striatal metabolome but not the behaviors, suggesting that the striatum underwent fundamental and perhaps irreversible changes due to iron deficiency during development.
Acknowledgments
The laboratory assistance of Katie Ennis and the editorial assistance of Eric Reese are gratefully acknowledged. The authors thank Betsy Lozoff, M.D., for the critical review of the manuscript.
Footnotes
Funded in part by NIH grants P01HD39386 (Betsy Lozoff, PI) and T32 MH073129 (K.L.W). The Center for Magnetic Resonance Research is in part supported by a center grant from the National Center for Regional Resources (RR08079) and the MIND Institute. The 9.4 tesla magnet is funded in part by a gift from the W. M. Keck Foundation.
- G
- gestational day
- GPC + PCho
- sum of glycerophosphorylcholine and phosphorylcholine
- ID
- iron-deficient
- IS
- iron-sufficient
- NAA
- N-acetylaspartate
- P
- postnatal day
Literature Cited
- 1.World Health Organization . Iron deficiency anemia: assessment, prevention and control: a guide for program managers. World Health Organization; Geneva: 2001. Available from: http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf. [Google Scholar]
- 2.Georgieff MK, Mills MM, Gordon K, Wobken JD. Reduced neonatal liver iron concentrations after uteroplacental insufficiency. J Pediatr. 1995;127:308–11. doi: 10.1016/s0022-3476(95)70317-9. [DOI] [PubMed] [Google Scholar]
- 3.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121:109–14. doi: 10.1016/s0022-3476(05)82554-5. [DOI] [PubMed] [Google Scholar]
- 4.Walter T, De Andrac I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics. 1989;84:7–17. [PubMed] [Google Scholar]
- 5.Pollitt E, Hathirat P, Kotchabhakdi NJ, Missell L, Valyasevi A. Iron deficiency and educational achievement in Thailand. Am J Clin Nutr. 1989;50:687–96. doi: 10.1093/ajcn/50.3.687. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 7.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–66S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 9.Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: involvement of dopamine-opiate system. Cell Mol Biol. 2000;46:491–500. [PubMed] [Google Scholar]
- 10.Lozoff B, Brittenham G, Viteri FE, Urrutia JJ. Behavioral abnormalities in infants with iron deficiency anemia. In: Pollitt E, Leibel RL, editors. Iron deficiency: brain biochemistry and behavior. Raven Press; New York: 1982. pp. 183–193. [Google Scholar]
- 11.Lozoff B, Wolf AW, Jimenez E. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996;129:382–9. doi: 10.1016/s0022-3476(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 12.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–32. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff M, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor JR, Menzies SL. Altered cellular distribution of iron in the central nervous system of myelin deficient rats. Neuroscience. 1990;34:265–71. doi: 10.1016/0306-4522(90)90320-4. [DOI] [PubMed] [Google Scholar]
- 15.deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 17.Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effects of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Dev Brain Res. 2005;156:202–9. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Schallert T, Petrie BF, Whishaw IQ. Neonatal dopamine depletion: spared and unspared sensorimotor and attentional disorders and effects of further depletion in adulthood. Psychobiology. 1989;17:386–96. [Google Scholar]
- 19.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 20.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126:693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 21.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–11. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 22.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, de Ungria M, Sullivan D, Wu P, Wobken JD, Nelson CA. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. J Nutr. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 26.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 27.Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesiondependent patterns of recovery in rats. Exp Neurol. 2005;191:310–7. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci. 1998;18:2777–87. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–62. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- 30.Dallman PR. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- 31.Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 32.Plaschke K, Yun SW, Martin E, Hoyer S, Bardenheuer HJ. Interrelation between cerebral energy metabolism and behaviour in a rat model of permanent brain vessel occlusion. Brain Res. 1999;830:320–9. doi: 10.1016/s0006-8993(99)01427-4. [DOI] [PubMed] [Google Scholar]
- 33.Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–74. [PubMed] [Google Scholar]
- 34.Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, O’Gorman E, Ruck A, Brdiczka D. Some new aspects of creatine kinase (CK):compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors. 1998;8:229–34. doi: 10.1002/biof.5520080310. [DOI] [PubMed] [Google Scholar]
- 35.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–45. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Gruetter R. In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int. 2002;41:143–54. doi: 10.1016/s0197-0186(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, Billups B, Rossi D, Sarantis M, Hamann M, Attwell D. The role of glutamate transporters in glutamate homeostasis in the brain. J Exp Biol. 1997;200:401–9. doi: 10.1242/jeb.200.2.401. [DOI] [PubMed] [Google Scholar]
- 38.Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann N Y Acad Sci. 2003;1003:226–40. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- 39.Ill AM, Mitchell TR, Neely EB, Connor JR. Metabolic analysis of mouse brains that have compromised iron storage. Metab Brain Dis. 2006;21:77–87. doi: 10.1007/s11011-006-9022-5. [DOI] [PubMed] [Google Scholar]
- 40.Bluml S, Seymour KJ, Ross BD. Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31) P MRS in in vivo human brain. Magn Reson Med. 1999;42:643–54. doi: 10.1002/(sici)1522-2594(199910)42:4<643::aid-mrm5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 42.Burri R, Steffen C, Herschkowitz N. N-acetyl-L-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci. 1991;13:403–11. doi: 10.1159/000112191. [DOI] [PubMed] [Google Scholar]
- 43.Catani M, Mecocci P, Tarducci R, Howard R, Pelliccioli GP, Mariani E, Metastasio A, Benedetti C, Senin U, Cherubini A. Proton magnetic resonance spectroscopy reveals similar white matter biochemical changes in patients with chronic hypertension and early Alzheimer’s disease. J Am Geriatr Soc. 2002;50:1707–10. doi: 10.1046/j.1532-5415.2002.50465.x. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78:736–45. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- 45.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002;82:736–54. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 46.Shonk TK, Moats RA, Gifford P, Michaelis T, Mandigo JC, Izumi J, Ross BD. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195:65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- 47.Yu GS, Steinkirchner TM, Rao GA, Larkin EC. Effect of prenatal iron deficiency on myelination in rat pups. Am J Pathol. 1986;125:620–4. [PMC free article] [PubMed] [Google Scholar]
- 48.Connor JR, Benkovic SA. Iron regulation in the brain: histochemical, biochemical, and molecular considerations. Ann Neurol. 1992;32(Suppl):S51–61. doi: 10.1002/ana.410320710. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–9. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 50.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68:683–90. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 51.DeRegnier RA, Nelson CA, Thomas KM, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137:777–84. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 52.Morris CM, Candy JM, Oakley AE, Bloxham CA, Edwardson JA. Histochemical distribution of non-haem iron in the human brain. Acta Anat (Basel) 1992;144:235–57. doi: 10.1159/000147312. [DOI] [PubMed] [Google Scholar]
- 53.Exposito I, Del Arco A, Segovia G, Mora F. Endogenous dopamine increases extracellular concentrations of glutamate and GABA in striatum of the freely moving rat: involvement of D1 and D2 dopamine receptors. Neurochem Res. 1999;24:849–56. doi: 10.1023/a:1020901929419. [DOI] [PubMed] [Google Scholar]
- 54.Lin JY, Dubey R, Funk GD, Lipski J. Receptor subtype-specific modulation by dopamine of glutamatergic responses in striatal medium spiny neurons. Brain Res. 2003;959:251–62. doi: 10.1016/s0006-8993(02)03757-5. [DOI] [PubMed] [Google Scholar]
- 55.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 56.O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–35. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- 57.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 58.West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- 59.Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]