FIGURE 2.
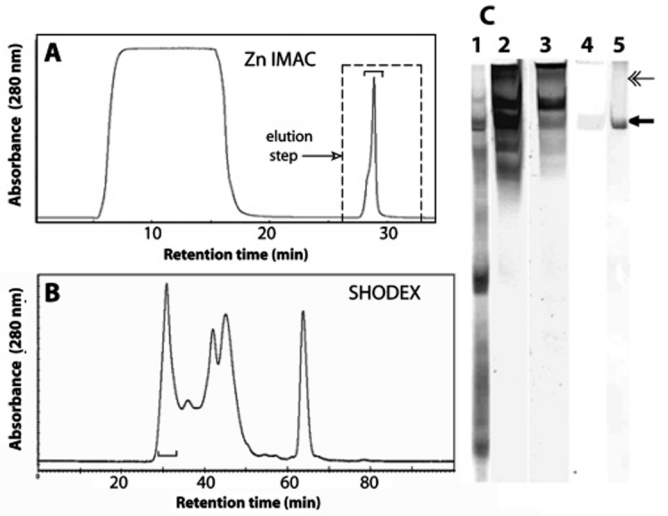
mcfp-4 from adhesive plaques. (A) Immobilized metal affinity chromatography (IMAC) of Mcfp-4 with a chelating column charged with Zn2+. Protein was eluted with a stepped increase to pH 3. (B) Gel filtration chromatography on Shodex 803 of the eluted IMAC-binding fractions. His-rich fractions are denoted with a bracket. (C) Acid urea-polyacrylamide gel electrophoresis of the key steps in mcfp-4 purification: crude soluble plaque extract from plaques stained with Coomassie blue R-250 (lane 1), IMAC column binding fractions stained in parallel for protein with CBR-250 (lane 2), Dopa with NBT in glycinate (lane 3), histidine with Pauly’s reagent (lane 4), and Shodex-purified mcfp-4 (arrow) stained with CBR-250 (lane 5). The double-headed arrow marks an internal calibrant, the α chain of type I collagen.
