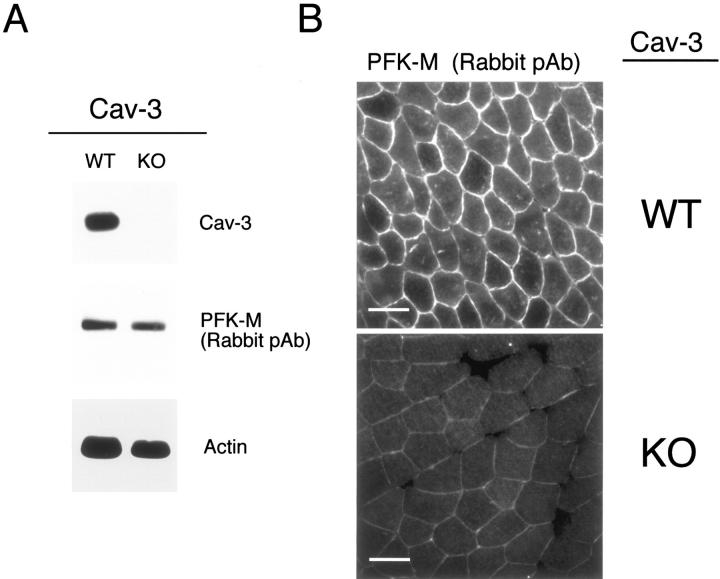
Figure 14.
In skeletal muscle tissue from Cav-3(−/−) mice, PFK isoforms show normal expression levels but are mislocalized. A: Western blot analysis. Protein lysates were prepared from skeletal muscle biopsies of WT and Cav-3(−/−) mice [knockout (KO)] and separated by SDS-PAGE. The blots were subsequently subjected to immunoblotting using a rabbit polyclonal anti-PFK-M antibody that cross-reacts with all three PFK isoforms. Note that PFK-M is expressed at normal levels in the skeletal muscle tissue of Cav-3-deficient mice as compared with WT control littermate mice. Equal loading was assessed by immunoblotting with a monoclonal antibody directed against actin (clone AC-40). B: Immunohistochemistry. Skeletal muscle tissue frozen sections were prepared from muscle biopsies of WT and Cav-3 (−/−) mice (KO) and were immunostained with a rabbit polyclonal anti-PFK-M antibody that cross-reacts with all of the three PFK isoforms. PFK-M shows both an intracellular and membrane bound distribution in WT skeletal muscle fibers. In contrast, note that the absence of Cav-3 impedes the recruitment of PFK-M to the sarcolemma in Cav-3-deficient skeletal muscle. Also, in Cav-3(−/−) muscle, the overall intensity of immunostaining for PFK-M appears reduced; this is mostly likely because of that fact that PFK-M is soluble in the absence of Cav-3 (not membrane bound) and, as a consequence, is partially washed away during the immunostaining procedure (see Figure 15B ▶ ). In support of this assertion, total PFK-M levels are unaffected in Cav-3-null mice as determined by Western blot analysis (A) (see also Figure 15A ▶ ). Scale bars, 50 μm.