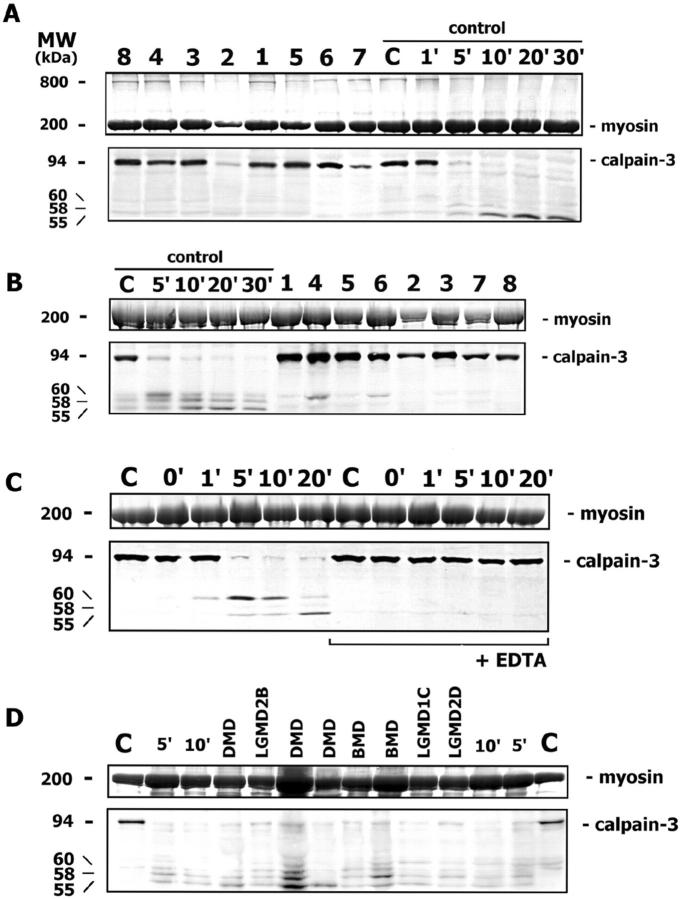
Figure 3.
A: Calpain-3 Western blotting in muscle biopsy sections incubated in loading buffer shows that all mutant patients (1 to 8) have calpain-3 of an amount comparable with the normal control (C), as determined by myosin in the posttransfer Coomassie blue-stained gel. The calpain-3 autocatalytic activity is tested in control muscle after different incubation times (from 1′ to 30′) in saline solution; the full-length calpain-3 band (94 kd) almost disappears after 5 minutes, and additional degradation bands at lower molecular weight (60, 58, and 55 kd) appear progressively. In the posttransfer Coomassie blue-stained gel, a high molecular-weight protein of about 800 kd is present in patient and control samples, where calpain-3 autocatalysis does not occur, and it disappears progressively during autolysis. B: The assay of calpain-3 autocatalytic activity is conducted in control muscle after various incubation times (from 5′ to 30′) and in patient samples (1 to 8) after 5 minutes. While in the control muscle the full-length calpain-3 almost disappears after 5 minutes and degradation bands appear progressively, in all patient samples the full-length calpain-3 is still present at an amount comparable with the non-autocatalyzed normal control. C: While an almost complete calpain-3 autocatalysis occurs in control muscle after 5 minutes of incubation, and degradation bands appear progressively, the addition of 10 mmol/L EDTA to the saline solution blocks the autocatalytic calpain-3 activity at all times. D: The calpain-3 autocatalytic activity is tested in muscle disease controls (DMD, BMD, LGMD2B, LGMD1C, LGMD2D) after 5 minutes of incubation: the full-length calpain-3 almost disappears in all patients and in control muscle after different incubation times (5′ and 10′), and degradation bands are present.