Figure 2.
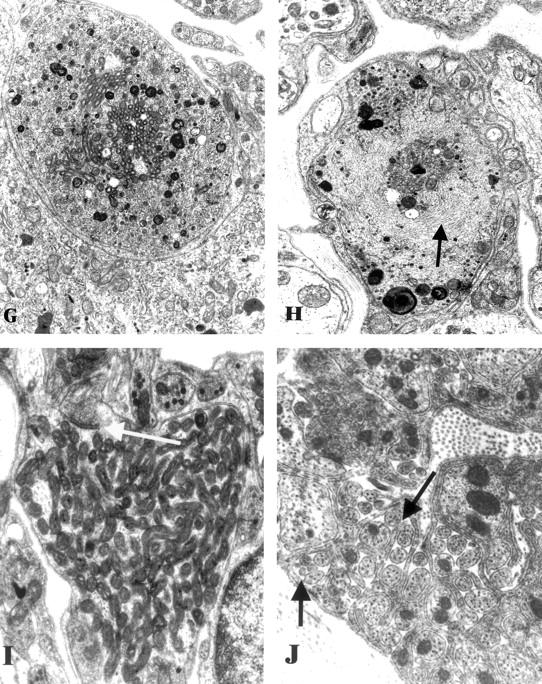
Ultrastructural appearance of the SMG-CG of 3 to 5 week diabetic NOD and non-diabetic control mice. A and B: Numerous dystrophic neurites (arrows, A) are scattered through the ganglionic neuropil and perineuronal space in the diabetic NOD mouse SMG-CG in comparison to the appearance of the normal ganglion of non-diabetic NOD mouse sibling (B). Original magnifications: ×980 (A); ×1950 (B). C and D: The most common ultrastructural appearance of dystrophic neurites in the diabetic NOD mouse consists of dilatations containing tubulovesicular elements. The contours of the sympathetic perikaryon adjacent to a large dystrophic element (arrow, C) are distorted but the neuron appears otherwise normal. A dystrophic neurite containing large numbers of tubulovesicular elements (D) is separated from the adjacent neuronal cell body (N) by satellite cell processes (arrows) along part of its circumference. Original magnifications: ×2600 (C); ×13,000 (D). E and F: Markedly enlarged neurites containing nearly pure collections of mitochondria represent a second major category of neuritic dystrophy Original magnifications: ×7800 (E); ×19,500 (F). G and H: Dystrophic neurites may also contain a variety of admixed organelles including mitochondria, tubulovesicular elements, and dense bodies (G) or neurofilaments (arrow, H). Original magnifications: ×15,600 (G and H). I: Dystrophic neurites occasionally exhibit synaptic specializations (arrow). Original magnification: ×24,200. J: The ganglionic neuropil may contain large numbers of axonal sprouts, suggesting a regenerative component. Original magnification: ×29,000.
