Figure 2. Association of 11E6 and 16E6 with E6AP in mammalian cells. A. Immune precipitation of FLAG-tagged 11E6 and 16E6 co-precipitates E6AP.
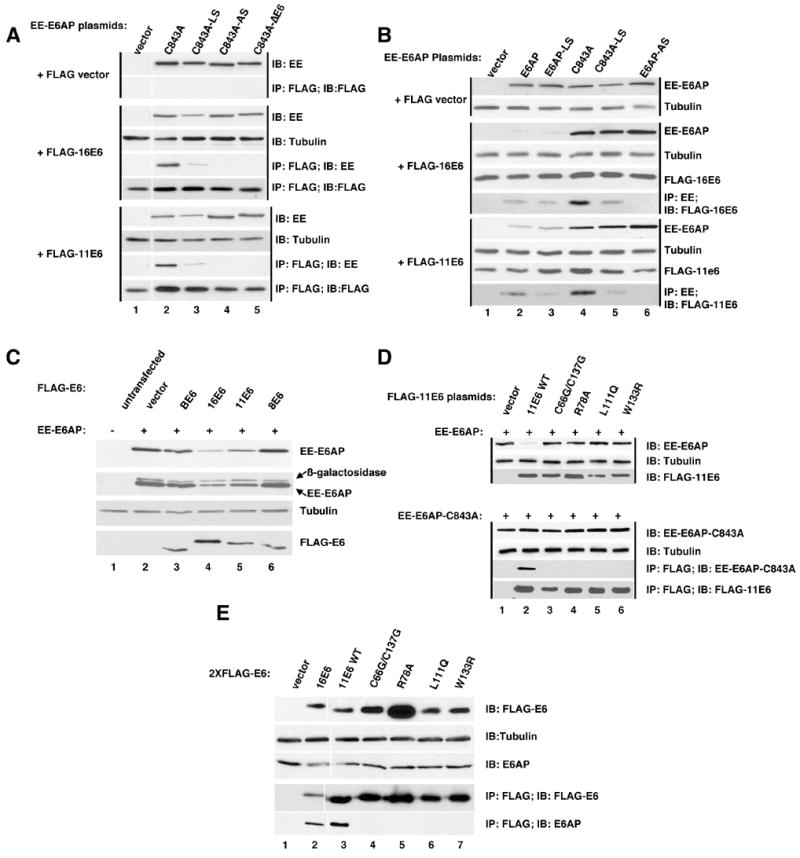
The indicated plasmids were transiently overexpressed in CV-1 cells by vaccinia pTM1 transfection, protein equalized NP40 soluble lysates prepared and either analyzed by immunoblot with the indicated antibodies or immune precipitated with antibodies directed to indicated epitope tags (Flag or EE) as previously described (Cooper et al., 2003). Black vertical lines group samples transfected with either FLAG vector, FLAG-16E6, or FLAG-11E6. A white vertical line between lanes 1 and 2 indicates the position of a lane excised from the figure. B. Immune precipitation of E6AP co-precipitates 11E6 and 16E6. Performed as in A. Black vertical lines group samples transfected with either FLAG vector, FLAG-16E6, or FLAG-11E6. C. Reduction of E6AP expression by co-expression of 11E6 and 16E6. The indicated plasmids were co-expressed in CV-1 cells as in parts A and B with a constant amount of beta-galactosidase included as an internal expression control. Protein normalized samples from NP40 lysed cells were probed with antibodies first for the EE tag on E6AP, then beta-galactosidase, then cellular tubulin as a loading control and finally FLAG-E6. D. 11E6 mutants defective for HPV-11 plasmid maintenance fail to interact with E6AP. In two parallel sets of transfections either EE-E6AP or EE-E6AP-C843A (mutated in ubiquitin ligase activity)were co-transfected with 11E6 wild-type (WT) or the indicated 11E6 mutants. Mutant C66G/C137G are in the zinc binding motif, L111Q and W133R are in conserved buried residues and R78A was undetermined in location in a recent structural model of E6 proteins (Nomine et al., 2006). Cells transfected with EE-E6AP were harvested in SDS lysis buffer and analyzed for expression in the upper three panels. Cells transfected with EE-E6AP-C843A were lysed in NP40 lysis buffer and protein content equalized, with a portion analyzed for EE-E6AP-C843A and tubulin expression in the lysate and the remainder immune precipitated with antibody to FLAG. Immunoblots were performed with the indicated antibodies. E. 11E6 and 16E6 associate with E6AP when stably expressed in C33A cells. C33A cells were retrovirally transduced with 2X-FLAG-tagged vector, 16E6, 11E6 or the indicated 11E6 mutants. 5% of clarified and protein equalized NP40 lysates from 6×107 pooled drug resistant cells 10 passages after completion of drug selection were analyzed by immunoblot for the expression of FLAG-E6 (top panel), tubulin (second panel) and E6AP (third panel from top). The remaining lysate was immune precipitated with rabbit polyclonal antibody to FLAG epitope and washed precipitates were analyzed for the expression of FLAG-E6 and associated E6AP using mouse monoclonal antibodies to FLAG and E6AP.
