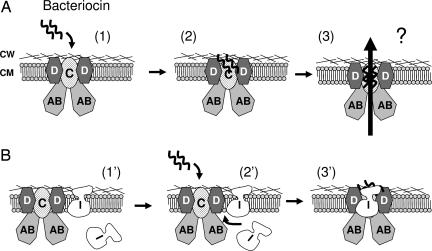
Fig. 5.
Model of target cell killing and immunity for lactococcin A and probably also for lactococcin B and some pediocin-like bacteriocins. (A) The bacteriocin employs IIC and IID of the man-PTS as a receptor on target cells (states 1 and 2). After binding, the bound bacteriocin somehow triggers permeabilization of the membrane (state 3), causing leakage of cellular components and, eventually, cell death. Although the exact nature of the pores is unknown, it is conceivable that they are formed either by oligomerized bacteriocin molecules or by disruption of the man-PTS. (B) In immune cells without bacteriocin production (e.g., the clone B268), the immunity protein (I) is not tightly associated with the man-PTS (state 1′). Only when exogenous bacteriocin is added to the culture medium (state 2′), the immunity protein approaches and binds strongly the target proteins (IIC and IID). The cells are thereby protected, probably through the blocking of the bound bacteriocin molecules, from advancing to the subsequent steps that lead to cell death (state 3′). In bacteriocin-producing cells (e.g., B244), the cognate immunity protein is tightly associated with the bacteriocin-bound receptor proteins to protect cells from being killed (state 3′). In all cases, the cytoplasmic component IIAB may form contact(s) with its membrane-located partners without being involved in the receptor-function. CW, cell wall; CM, cytoplasmic membrane. The man-PTS unit is shown with a stoichiometric ratio of 2:1:2 for IIAB/IIC/IID, based on the analogy to the E. coli counterpart (42).