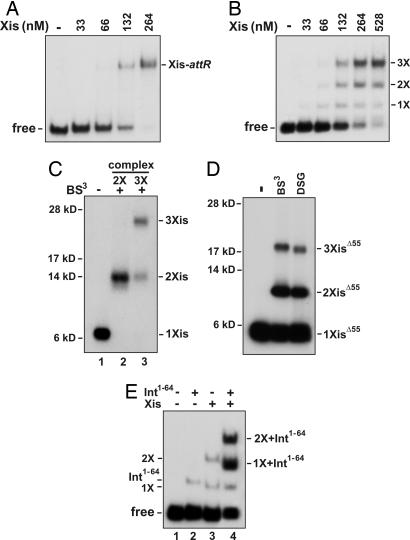
Fig. 2.
A trimer of Xis cooperatively binds DNA. (A) Cooperative Xis binding to long attR DNA fragments. Increasing concentrations of Xis were incubated with a 32P-labeled attR fragment (−220 to +43) and electrophoresed in a native polyacrylamide gel. (B) Xis binding to short attR DNA fragments (−104 to −51) exhibits reduced cooperativity. 1X, 2X, and 3X represent DNA complexes postulated to contain one, two, and three Xis monomers, respectively. (C) Cross-linking of Xis–attR complexes. 32P-labeled XisHMK was incubated with fluorescein-labeled attR DNA (−104 to −54), subjected to cross-linking with BS3, and electrophoresed in a native gel as in B. Xis complexes representing 2X and 3X were extracted from the native gel and subjected to SDS/PAGE. The number of cross-linked Xis monomers in each product band is based on their apparent molecular mass. (D) Cross-linking of Δ55Xis–attR complexes. Protocol was the same as in C except that 32P-labeled Δ55XisHMK was subjected to cross-linking with BS3 (11-Å spacer) or DSG (8-Å spacer), and the cross-linked products were extracted from the dominant slowest migrating complex. (E) Binding of Int1–64 and two Xis protomers to attR fragments missing the X2 site. Xis (0.53 μM) and HisInt1–64 (3 μM) were incubated with a 32P-labeled 45-bp probe (−121 to −77) containing the P2 and X1 binding sites as designated and electrophoresed in a native polyacrylamide gel. The identities of each of the bands based on their electrophoretic mobilities are denoted. The sequences of the oligonucleotide substrates are given in SI Fig. 6.